Reviewed by Danielle Ellis, B.Sc.Jun 9 2022
For the first time, researchers have deciphered the atomic structure of a protein that transports one of the body’s most vital neurotransmitters into neurons.
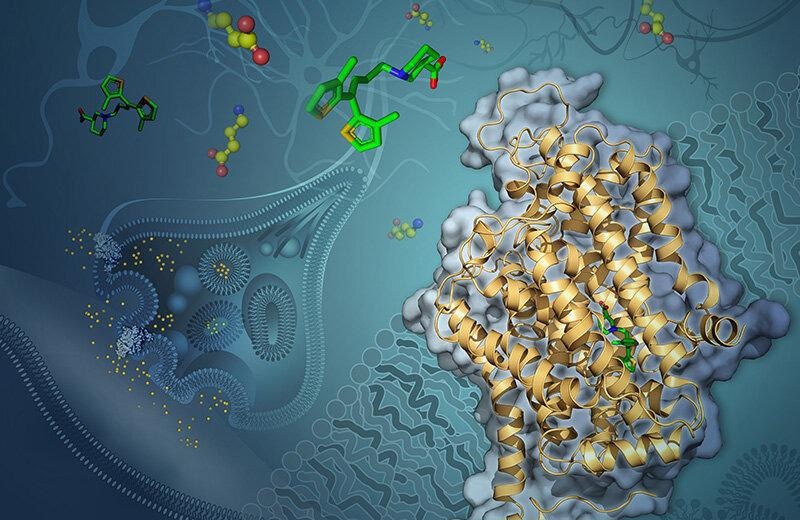 Artist’s rendering of GABA transporter 1 (GAT-1) embedded in a neuron’s membrane. Tiagabine (green) blocks GAT-1 from shuttling the neurotransmitter GABA (yellow and red) into the cell. Image Credit: Yekaterina Kadyshevskaya
Artist’s rendering of GABA transporter 1 (GAT-1) embedded in a neuron’s membrane. Tiagabine (green) blocks GAT-1 from shuttling the neurotransmitter GABA (yellow and red) into the cell. Image Credit: Yekaterina Kadyshevskaya
The scientists opened new avenues for improving drugs for a broad range of debilitating illnesses, such as epilepsy, bipolar disorder, schizophrenia, Parkinson’s and Huntington’s diseases, anxiety, and autism spectrum disorder, by understanding the shape of this transporter protein—one of the smallest proteins ever rectified.
The study was published on June 8th, 2022, in the journal Nature.
Neurons communicate with one another by sending neurotransmitters via synapses, which are spaces between them. The neurotransmitter GABA (short for gamma-amino butyric acid) is one of the most common in the brain.
When a neuron releases GABA and sends it across a synapse to a neighboring neuron, GABA inhibits the receiving cell’s activity.
However, there are situations when not enough GABA reaches the receiving neuron, causing it to become overactive and send too many electrical impulses. This can have a variety of negative consequences, like seizures.
Apparently, medications that block a protein called GAT-1 can assist (short for GABA transporter 1). GAT-1 is in charge of reintroducing released GABA into the generating neuron.
Tiagabine (brand name Gabitril), a GAT-1 inhibitor, inhibits GABA recycling, leaving more GABA in the synapse to diminish receiving neuron activity.
Although tiagabine is effective, the exact mechanism by which it inhibits GABA recycling by interacting with GAT-1 is unknown. Knowing how they function could help scientists develop more effective medications in the future.
Scientists at the USC Dornsife College of Letters, Arts, and Sciences employed very sophisticated cryogenic electron microscopy, also known as cryo-EM, to unravel the enigma and discover exactly how tiagabine attaches to GAT-1. The method entails freezing molecules at extremely low temperatures, close to the point at which atoms and molecules cease to move, and then picturing them with an electron microscope.
The USC Michelson Center for Convergent Bioscience recently presented its new cryo-EM laboratory in the Core Center of Excellence in Nano Imaging.
Cornelius Gati, the study’s lead author, and a group of USC Dornsife researchers observed GAT-1 in complex with tiagabine and used cryo-EM to show how the two interact.
Previous understanding of the inhibitor (tiagabine) was purely based on biochemical studies, which do not provide any details at the atomic scale. We can now pinpoint specific parts of the drug that interact with the protein.”
Cornelius Gati, Assistant Professor, Biological Sciences and Chemistry, USC Dornsife College of Letters, Arts, and Sciences
This new revelation, according to Gati, points to a previously undiscovered process of GAT-1 inhibition involving a change in the protein’s overall structure when tiagabine binds to it.
The study’s extremely detailed insights could aid researchers in improving medications or developing novel remedies for disorders involving GABA-controlled neurons.
Gati went on to say that the findings suggest new research directions, stating “These findings have direct implications on pharmacology as a whole, not only with respect to treating epilepsy but many other diseases.”
We have a feeling that this newly revealed mechanism is much more common than is currently thought, so we will investigate this first by using structurally similar inhibitors, and then expand our search to other molecules.”
Cornelius Gati, Assistant Professor, Biological Sciences and Chemistry, USC Dornsife College of Letters, Arts, and Sciences
Gati also mentioned that utilizing cryo-EM to resolve the structure of the interacting molecules was incredibly difficult—GAT-1 is one of the tiniest proteins recovered using the method, making it tough to visualize even with such sophisticated technology.
Their breakthrough, he added, will inspire researchers at USC and other institutions to figure out the structures of additional difficult membrane proteins, resulting in a better knowledge of drug–protein interactions and more effective therapies.
Source:
Journal reference:
Motiwala, Z., et al. (2022) Structural basis of GABA reuptake inhibition. Nature. doi.org/10.1038/s41586-022-04814-x.