Researchers at Johns Hopkins Medicine have reported that they have interrogated the atomic structure of proteins, adding to proof that the wobbles, shakes, and quivers of proteins play an important role in their ability to operate. The study’s findings may aid researchers in developing new drugs that can adjust or destabilize the intricate “dances” of proteins to change their functions.
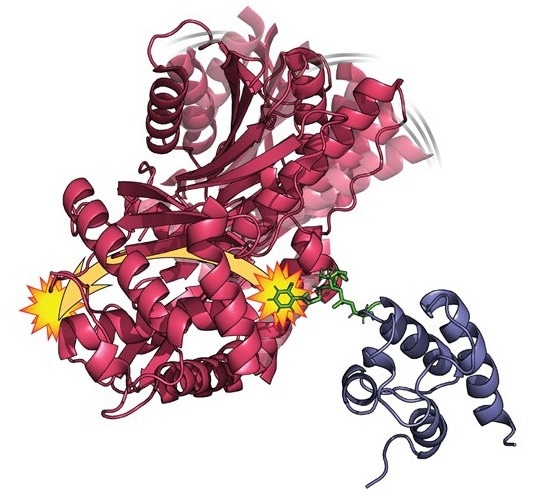 Structural illustration of the HMWP2 enzyme domains, highlighting two interacting domains (shown in red and blue), the two remote binding sites (yellow stars), and the modification of one domain (green). Image Credit: Kenny Marincin and Dominique Frueh, Johns Hopkins
Structural illustration of the HMWP2 enzyme domains, highlighting two interacting domains (shown in red and blue), the two remote binding sites (yellow stars), and the modification of one domain (green). Image Credit: Kenny Marincin and Dominique Frueh, Johns Hopkins
The findings of the researchers’ experiments were published in Science Advances on July 15th, 2022.
Proteins are organic compounds with DNA blueprints that serve as the “business ends” of biology, forming the structural components of tissues, alongside enzymes, which orchestrate chemical changes inside cells.
Though researchers have long known that proteins wiggle and move, the importance of this “dancing” act has been discussed, according to Dominique Frueh, PhD., associate professor of biophysics and biophysical chemistry at the Johns Hopkins University School of Medicine.
The way proteins engage with the right partner at the right time — essentially, how they communicate—is very important for understanding their function, and we have found that protein wiggles are critical for this communication.”
Dominique Frueh, PhD, Associate Professor, Biophysics and Biophysical Chemistry, School Of Medicine, Johns Hopkins University
Frueh’s team investigated the wiggling action of the HMWP2 protein, which is a type of enzyme known as nonribosomal peptide synthetases. These enzymes are composed of many domains, or unique regions, which collaborate like an assembly line to produce complex natural products from simple chemicals.
These organic ingredients, like bacitracin, which is found in topical antibiotic ointments, frequently have pharmaceutical qualities. In the case of HMWP2, the product is yersiniabactin, a molecule that hunts down iron molecules for bacteria such as E. coli, which causes urinary tract infections, and Yersinia pestis, which causes bubonic plague.
According to Frueh, recognizing how protein domains interact with one another could allow researchers to adjust the domains to develop new chemicals.
In a broad sense, the researchers discovered that the widespread wriggling of one domain in the HMWP2 enzyme initiates a process that allows the domain to connect with multiple partner domains at the same time.
To evaluate the importance of protein movement, the researchers used nuclear magnetic resonance (NMR) spectroscopy to monitor the movement of one of HMWP2’s domains down to each individual atom in the molecule. NMR spectroscopy is a device that uses potent magnetic fields to investigate the molecular surroundings of nuclei within the centers of atoms.
Even though NMR is frequently used to ascertain small protein structures, tracking movements within large proteins is challenging with the device.
Frueh’s team, which included NMR scientist Subrata Mishra, PhD, graduate student Kenneth Marincin, and postdoctoral fellow Aswani Kancherla, PhD, used nitrogen-15 and carbon-13—naturally produced forms of nitrogen and carbon—to badge two domains of the HMWP2 enzyme.
This technique was also used to monitor the change in direction of one domain when a second domain was altered, as happens when the enzyme makes its natural product.
We found that the two domains would only bind to one another as the second domain gets modified, which means they would only engage as needed for making the product and avoid wasting time together when the second domain is not modified. Somehow, the first domain is able to sense when the second domain is modified, and we sought to investigate whether motions played a role in this recognition process.”
Dominique Frueh, PhD, Associate Professor, Biophysics and Biophysical Chemistry, School Of Medicine, Johns Hopkins University
They also discovered motion variations throughout the entire domain identified with carbon-13c, not just where it unites to the second domain but also at a second, remote binding site used by a third domain.
On an atomic level, Frueh believes these two HMWP2 sites are “far” apart—about 40 billionths of a meter. The researchers were particularly interested in how they communicated despite their distance.
To demonstrate that movement aided interaction with the remote site and sense of the second domain alteration, the researchers genetically altered HMWP2 proteins with a mutation that took place in a location on the domain far from the two sites recognized by the researchers. As a result, the mutation did not directly prevent the sites from interacting with other domains.
“We found that the protein domain was structurally stable, but all of its movement was hindered,” says Frueh. According to scientists, the mutated protein’s lack of mobility impaired its potential to adhere to other domains even when they were altered, illustrating that the motions within the protein were required for the domains to work together.
According to Frueh, researchers could use extensive knowledge of protein movement to develop new drugs that do not attack a protein’s natural active site but rather stop its movement to inactivate it. According to him, such an approach could provide more leeway in designing drugs with fewer undesirable side effects.
Researchers are investigating how computation and machine learning can enhance insight and prediction of protein movement, according to Frueh.
Source:
Journal reference:
Mishra, S. H., et al. (2022) Global protein dynamics as communication sensors in peptide synthetase domains. Science Advances. doi.org/10.1126/sciadv.abn6549.