For the first time, scientists at the Centre for Genomic Regulation (CRG) have discovered a DNA sequence that is essential for pancreatic development and function.
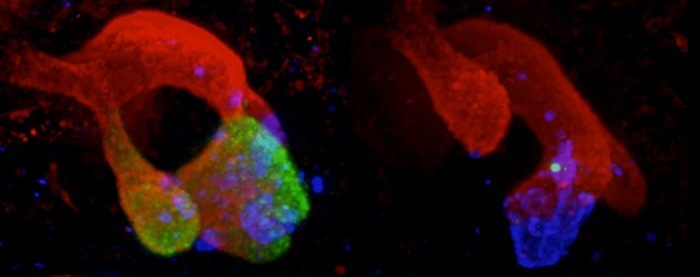 Mice with a deletion in the pancreas agenesis enhancer do not express PTF1A (green) in embryonic multipotent pancreatic progenitors, leading to underdeveloped pancreas and insulin-deficient diabetes. Immunofluorescence of embryonic pancreatic buds from control (left) and enhancer-deleted (right) mice stained for PTF1A (green), PDX1 (red), glucagon (blue). Image Credit: Miguel Angel Maestro/CRG/Developmental Cell
Mice with a deletion in the pancreas agenesis enhancer do not express PTF1A (green) in embryonic multipotent pancreatic progenitors, leading to underdeveloped pancreas and insulin-deficient diabetes. Immunofluorescence of embryonic pancreatic buds from control (left) and enhancer-deleted (right) mice stained for PTF1A (green), PDX1 (red), glucagon (blue). Image Credit: Miguel Angel Maestro/CRG/Developmental Cell
Patients who have a DNA mutation known as EnhP experience pancreas abnormalities. It is the most blatant instance of a hereditary disease that has not yet been proven to be brought on by mutations that alter the DNA sequence of a gene.
Monogenic disorders are conditions brought on by mutations in a single DNA sequence, such as Huntington’s disease or sickle cell anemia. Such mutations usually cause a protein-coding gene to malfunction. Instead of affecting a single gene in this instance, the mutations in EnhP affect a single “enhancer.”
Thousands of DNA fragments known to function as enhancers are found in human genomes. As switches, these enhancer DNA sequences activate each gene's transcription in the appropriate tissues.
EnhP is not the only enhancer deficiency that results in disease, according to the study’s authors, which was just published in Developmental Cell. In many individuals when laboratory studies have not revealed causative gene alterations, mutations in enhancers could be the source of a monogenic disease.
If the role of enhancers in disease is recognized, perceptions of existing medical practices might shift.
Clinical genetics is shifting from a focus on sequencing protein-coding genes to sequencing whole genomes. It is now theoretically possible to discover disease-causing mutations that lie outside of traditional areas of the genome, although it is still challenging to discern which parts of the genome are truly vulnerable to mutations.”
Dr Jorge Ferrer, Study Senior Author and Coordinator, Medical Genomics Transversal Program, Centre for Genomic Regulation
EnhP was first identified by the researchers while they were researching 10 distinct families' cases of developmental abnormalities.
They discovered in collaboration with a team in Exeter, United Kingdom, that pancreatic agenesis, a rare congenital condition that results in the loss of pancreatic tissue and neonatal diabetes, was most frequently caused by mutations in the enhancer.
In this study, researchers capitalized on their earlier findings to understand why this specific enhancer is susceptible to mutations that cause sickness. For the purpose of researching the effects of the enhancer, the scientists genetically modified mice models using CRISPR.
Mice lacking both copies of EnhP have significantly underdeveloped pancreas at birth as well as diabetes with inadequate insulin production. Additionally, they looked into in vitro human stem cells.
They demonstrate how EnhP functions by accelerating the transcription of the pancreatic-associated transcription factor 1a gene, a neighboring gene (PTF1A). More specifically, the findings showed that EnhP’s sole function in the first cells that grow into the pancreas during fetal development is to activate a large group of enhancers that also control PTF1A.
When PTF1A transcription is turned on and these other enhancers are engaged, a series of molecular actions occur that result in the production of healthy pancreatic cells.
Dr Ferrer added, “We show that enhancers operate in a hierarchical manner, and this one sit straight at the top. This is a new concept, and it solves a paradox of how mutations in a single enhancer can be catastrophic despite the existence of multiple other enhancers regulating the same gene.”
“This is not just about this particular enhancer or disease, there are probably many other enhancers with this particular function in the human genome. Finding them will help us understand which enhancers are vulnerable to mutations that cause various other monogenic diseases,” further stated Dr Ferrer.
The finding has implications for efforts to create beta cells that produce insulin in a lab setting. Although cell transplantation is a viable alternative for diabetes patients, there is a significant need for functioning cells from deceased donors.
An approach to solving this problem would be to grow beta cells in culture, but these cells hardly ever have the same functional characteristics as typical human beta cells. This is partially due to the lack of knowledge regarding the mechanisms necessary for appropriate differentiation.
Dr Ferrer concluded, “EnhP sparks a molecular program that is needed for proper formation of human beta cells. This knowledge can be harnessed to improve laboratory conditions to create beta cells.”
Source:
Journal reference:
Miguel-Escalada, I., et al (2022) Pancreas agenesis mutations disrupt a lead enhancer controlling a developmental enhancer cluster. Developmental Cell. doi:10.1016/j.devcel.2022.07.014