A groundbreaking study from the Icahn School of Medicine at Mount Sinai found that astronauts are more likely to experience mutations that could be connected to spaceflight and increase their lifetime risk of acquiring cancer and heart disease.
 Characteristics of Clonal Hematopoiesis (CH) Mutations. Image Credit: Mount Sinai Health System
Characteristics of Clonal Hematopoiesis (CH) Mutations. Image Credit: Mount Sinai Health System
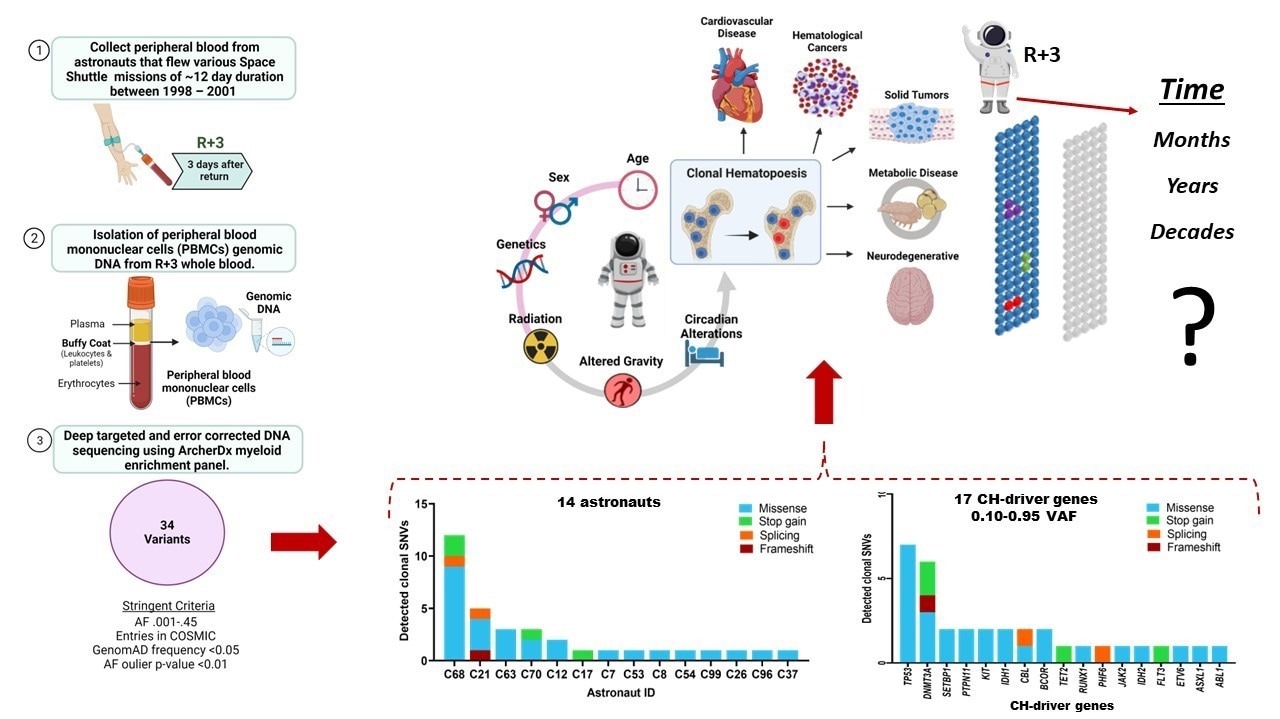
National Aeronautics and Space Administration (NASA) astronauts who flew space shuttle missions between 1998 and 2001 provided blood samples to a research team. In the blood-forming system (hematopoietic stem cells) of all 14 astronauts under study, they found somatic DNA alterations.
The research, which was published in the journal Nature Communications Biology, emphasizes the possibility that these mutations may be brought on by spaceflight and highlights the significance of routine blood testing for astronauts throughout their careers and during retirement to keep an eye on their health.
Somatic mutations, which cannot be passed on to offspring because they happen after conception and in cells other than sperm or egg cells, are mutations that occur in an individual. The overrepresentation of blood cells produced from a single clone, or clonal hematopoiesis (CH), was a characteristic of the mutations found in this study.
These mutations can result from cancer chemotherapy or radiation therapy and are frequently brought on by environmental factors including exposure to ultraviolet radiation or certain chemicals. CH has few telltale signs or symptoms; the majority of patients are discovered through genetic testing of their blood for other disorders. Although CH is not always a sign of illness, it is linked to a higher risk of blood cancer and cardiovascular disease.
Astronauts work in an extreme environment where many factors can result in somatic mutations, most importantly space radiation, which means there is a risk that these mutations could develop into clonal hematopoiesis.”
David Goukassian MD, Study Lead Author and Professor, Medicine (Cardiology), Cardiovascular Research Institute, Icahn School of Medicine, Mount Sinai
Dr Goukassian adds, “Given the growing interest in both commercial spaceflights and deep space exploration, and the potential health risks of exposure to various harmful factors that are associated with repeated or long-duration exploration space missions, such as a trip to Mars, we decided to explore, retrospectively, somatic mutation in the cohort of 14 astronauts.”
Astronauts who performed relatively brief (median 12 days) space shuttle flights between 1998 and 2001 made up the study’s subjects. Six of the 14 were on their first mission, the median age was about 42, and almost 85% of them were men.
White blood cells were only collected from the astronauts three days after landing; complete blood samples were taken 10 days prior to the journey and on the day of landing. The samples were kept for around 20 years at −80 ºC.
Researchers discovered 34 mutations in 17 CH-driver genes using DNA sequencing and in-depth bioinformatics studies. The most often altered genes were TP53, which makes a protein that suppresses tumor growth, and DNMT3A, which is typically mutated in acute myeloid leukemia.
However, the somatic mutation frequency in the genes that the researchers examined was less than 2%, which is the technical cutoff for somatic mutations in hematopoietic stem cells to be regarded as clonal hematopoiesis with uncertain potential (CHIP). CHIP is more prevalent in older people and is linked to a higher risk of cardiovascular disease as well as hematologic and solid cancers.
Although the clonal hematopoiesis we observed was of a relatively small size, the fact that we observed these mutations was surprising given the relatively young age and health of these astronauts. The presence of these mutations does not necessarily mean that the astronauts will develop cardiovascular disease or cancer, but there is the risk that, over time, this could happen through ongoing and prolonged exposure to the extreme environment of deep space.”
David Goukassian MD, Study Lead Author and Professor, Medicine (Cardiology), Cardiovascular Research Institute, Icahn School of Medicine, Mount Sinai
Dr Goukassian states, “Through this study, we have shown that we can determine the individual susceptibility of astronauts to develop disease related to their work without any implications that can affect their ability to do their work. Indeed, our studies demonstrate the importance of early and ongoing screening to assess that susceptibility.”
Our recommendation is that NASA, and its medical team, screen astronauts for somatic mutations and possible clonal expansion, or regression, every three to five years, and, not less importantly, well into their retirement years when somatic mutations may expand clonally and become CHIP.”
David Goukassian MD, Study Lead Author and Professor, Medicine (Cardiology), Cardiovascular Research Institute, Icahn School of Medicine, Mount Sinai
Exosomes are tiny, lipid-layered microscopic vesicles that form inside human body cells and are then released into the blood circulation, carrying information from their cells of origin that mirrors their intercellular condition. The group's research builds on earlier investigations that utilized the same samples to recognize predictive biomarkers in exosomes.
This property of exosomes may make them excellent biomarkers of health and/or disease and enable them to transmit data between cells throughout the body over considerable distances. The exosomes from astronauts that the investigators used to treat human heart cells had an impact on the biology of the vitamin D receptor, which is crucial for the health of the heart, skeletal muscles, and bones.
Additionally, they evaluated how space travel will affect mitochondrial DNA, which makes up the genome of the tiny organelles that power cells. Researchers discovered that astronauts’ blood had two to 350 times the average quantity of cell-free mitochondrial DNA circulating, which could cause oxidative stress and inflammation in other parts of the body.
“Through these studies, we have demonstrated the potential to assess the health risk of space flight among astronauts. What is important now is to conduct longitudinal retrospective and well-controlled prospective studies involving a large number of astronauts to see how that risk evolves based on continued exposure and then compare that data against their clinical symptoms, imaging, and lab results,” elaborated Dr Goukassian.
Dr Goukassian concludes, “That will enable us to make informed predictions as to which individuals are more likely to develop disease based on the phenomena we are seeing and open the door to individualized precision medicine approaches to early intervention and prevention.”
Source:
Journal reference:
Brojakowska, A., et al. (2022) Retrospective analysis of somatic mutations and clonal hematopoiesis in astronauts. Communications Biology. doi.org/10.1038/s42003-022-03777-z.