The DDX41 gene encodes the nuclear enzyme DEAD-box-type RNA helicase. Hematopoietic malignancies are caused by DDX41 mutations. However, the mechanism behind the development of this malignancy remains unknown. Researchers from Japan defined the functional importance of DDX41 in considerable detail.
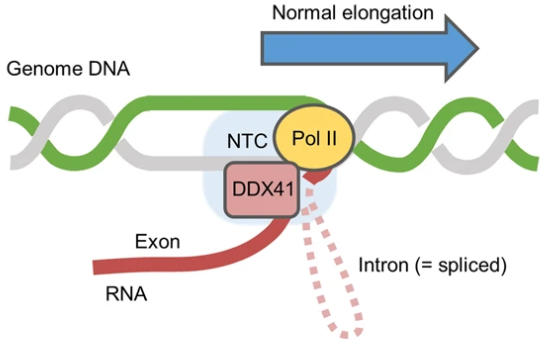 DDX41 regulates the pausing of RNA polymerase II during transcription, and resolves excess R-loop formation, thereby reducing replication stress and maintaining genomic stability. Image Credit: H. Matsui et.al., Leukemia, 2022.
DDX41 regulates the pausing of RNA polymerase II during transcription, and resolves excess R-loop formation, thereby reducing replication stress and maintaining genomic stability. Image Credit: H. Matsui et.al., Leukemia, 2022.
Their findings show that DDX41 plays critical roles in transcriptional processes, RNA splicing, and genomic integrity preservation. The results could help in the treatment of hematopoietic cancers.
DNA replication, transcription, and post-transcriptional modifications are critical biological processes mediated by a variety of enzymes. RNA helicases are a critical class of enzymes involved in a variety of transcriptional and post-transcriptional activities. Among these is the DEAD-box-type RNA helicase, which is mostly found in the nucleus and is encoded by the DDX41 gene.
Mutations in the DDX41 gene can cause malignancy of the hematopoietic cell progenitors (blood cell precursors), such as acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). However, the processes behind the development of cancer are incompletely understood.
To bridge this gap, a team of researchers headed by Prof. Hirotaka Matsui of Kumamoto University’s Graduate School of Life Sciences carried out a comprehensive investigation to uncover the detailed functional processes of DDX41.
Our research group is investigating the DDX41 gene, which is known to be one of the causative genes of AML/MDS. Focusing on this gene, we have analyzed the details of the damage caused to cells by the mutation of this gene.”
Hirotaka Matsui, Professor, Graduate School of Life Sciences, Kumamoto University
The observations, co-authored by several investigators, including Prof. Toshiya Inaba of Hiroshima University’s Research Institute for Radiation Biology and Medicine and Dr Akihiko Yokoyama of the National Cancer Center’s Tsuruoka Metabolomics Laboratory, were published on October 14th, 2022 in the journal Leukemia.
The researchers demonstrated that DDX41 plays a vital role in mRNA synthesis by binding to the 5′ splice site (SS) of the coding RNA in a sequence of cellular assays on the parental K562 cell line and the DDX41-mutant R525H-expressing cell line.
Further research indicated that DDX41 interacts with proteins involved in RNA splicing, a molecular process required for the production of mRNA. These findings highlight the relevance of DDX41 in transcriptional events.
The investigators looked into the effects in DDX41-knockdown cells, where the levels of DDX41 were lowered, to further evaluate the functional relevance of DDX41 and comprehend the mechanism behind malignancy development.
These investigations demonstrated that DDX41-knockdown cells experienced abnormalities in DNA replication and mitosis (the process by which cells divide). In the knockdown cells, the researchers also noticed an increase in the development of R-loops, which are structures made up of a DNA:RNA hybrid and an unpaired DNA.
The findings concur with those of investigations in which the DDX41 function was suppressed. There was moderate replication stress after inhibition, which ultimately led to cell cycle disruption due to a delayed G2-M transition, a critical checkpoint in the cell cycle. Moreover, the results indicated that DDX41 modulates the pausing of RNA polymerase II, which is required for effective transcription.
The findings offer light on DDX41’s functional relevance and indicate its role in genomic stability. The findings will contribute to the understanding of the processes behind the development of hematopoietic malignancies in people with DDX41 mutations, which may eventually contribute to the therapeutic domain.
In the future, we will elucidate detailed mechanisms by which hematopoietic malignancies develop. If we can establish a molecular target therapy, such malignancies will have greater potential for cure.”
Hirotaka Matsui, Professor, Graduate School of Life Sciences, Kumamoto University
Source:
Journal reference:
Shinriki, S., et al. (2022) DDX41 coordinates RNA splicing and transcriptional elongation to prevent DNA replication stress in hematopoietic cells. Leukemia. doi.org/10.1038/s41375-022-01708-9.