Evolutionarily young miniproteins are unique in humans, and scientists have recently found thousands of them.
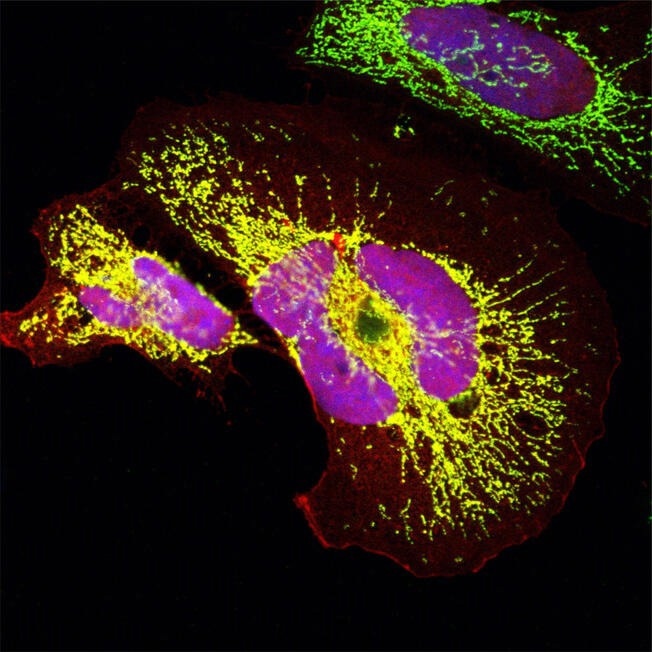 An evolutionarily young protein that arose de novo in Old World monkeys: The microprotein in the mitochondria (green) and in the nucleus (blue) was overexpressed in human cells. The yellow and pink areas show that the signal of the microprotein overlaps with the mitochondrial and nuclear signals. Image Credit: © Clara-Louisa Sandmann, Max Delbrück Center
An evolutionarily young protein that arose de novo in Old World monkeys: The microprotein in the mitochondria (green) and in the nucleus (blue) was overexpressed in human cells. The yellow and pink areas show that the signal of the microprotein overlaps with the mitochondrial and nuclear signals. Image Credit: © Clara-Louisa Sandmann, Max Delbrück Center
Published in the journal Molecular Cell, Norbert Hübner and collaborators from the BIH and other institutions explain the origins of such small proteins and describe that they likely impact significant cellular processes.
Every biologist knows that small structures could sometimes create a big impact: Millions of signaling molecules, hormones, and other biomolecules are agitated in the cells and tissues. This plays a leading role in several of the main processes taking place in human bodies.
Still, despite this knowledge, a particular class of proteins was neglected by biologists and physicians—their assumption being that since the proteins were so small and only discovered in primates, they were functionless and unimportant.
The breakthroughs made by Professor Norbert Hübner at the Max Delbrück Center and Dr Sebastiaan van Heesch at the Princess Máxima Center for Pediatric Oncology in the Netherlands altered this view a few years ago:
We were the first to prove the existence of thousands of new microproteins in human organs.”
Norbert Hübner, Head of the Lab, Genetics and Genomics of Cardiovascular Diseases, Max Delbrück Center
In a new study reported in the journal Molecular Cell, the team headed by Hübner and van Heesch currently explain how they systematically studied such miniproteins, and what they learned from them
“We were able to show which genome sequences the proteins are encoded in, and when DNA mutations occurred in their evolution,” explained Dr Jorge Ruiz-Orera, an evolutionary biologist in Hübner’s lab and one of the paper’s three lead authors, who work at the Max Delbrück Center and the German Center for Cardiovascular Research (DZHK).
Ruiz-Orera’s bioinformatic gene analyses disclosed that most of the human microproteins developed millions of years later in the evolutionary process compared to the larger proteins that are presently known to scientists.
Still, the huge age gap does not appear to avoid the proteins from “talking” to each other.
Our lab experiments showed that the young and old proteins can bind to each other—and in doing so possibly influence each other.”
Dr Jana Schulz, Study Lead Author and Researcher in Hübner’s Team, Max Delbrück Center
Hence, Schulz suspects that, opposite to long-held assumptions, microproteins play a main role in a range of cellular functions. Also, the young proteins may be heavily involved in evolutionary development as a result of the comparatively rapid “innovations and adaptations.” “It’s possible that evolution is more dynamic than previously thought,” states van Heesch.
Proteins only found in humans
The scientists were surprised to determine that the immensely younger microproteins could cooperate with the much older generation. This remark came from experiments executed using a biotechnical screening method that has been developed at the Max Delbrück Center in 2017.
In partnership with Dr Philipp Mertins and the Proteomics Platform, which the Max Delbrück Center operates collaboratively with the Berlin Institute of Health at Charité (BIH), the miniproteins were synthesized on a membrane and further made to incubate with a solution consisting majority of the proteins known to survive in a human cell.
Furthermore, advanced experimental and computer-aided analyses enabled the scientists to determine individual binding pairs.
If a microprotein binds to another protein, it doesn’t necessarily mean that it will influence the workings of the other protein or the processes that the protein is involved in.”
Dr Jana Schulz, Study Lead Author and Researcher in Hübner’s Team, Max Delbrück Center
But the potential to bind does indicate the proteins may impact each other’s functioning. Initial cellular experiments were performed at the Max.
Delbrück Center in partnership with Professors Michael Gotthardt and Thomas Willnow verifies this assumption. This results in Ruiz-Orera to doubt that the microproteins “could influence cellular processes that are millions of years older than they are because some old proteins were present in the very earliest life forms.”
Contrary to the known, old proteins that are encoded in the genome, the majority of the microproteins arose more or less “out of nowhere—in other words, out of DNA regions that weren’t previously tasked with producing proteins,” states Ruiz-Orera.
Hence, microproteins did not take the “conventional” and much simpler route of being copied and derived from the present versions. Also, because such small proteins only emerged at the time of human evolution, they are lacking from the cells of the majority of other animals, like fish, mice, and birds. But these animals have been discovered to hold their collection of young and small proteins.
The smallest proteins so far
At the time of their work, the scientists also found the smallest human proteins determined so far.
“We found over 200 super-small proteins, all of which are smaller than 16 amino acids,” says Dr Clara Sandmann, the study’s third lead author.
Amino acids are known to be the sole building blocks of proteins. Sandmann states that this increases the question of how small a protein could be—or rather, how big it should be to be able to function. Generally, proteins consist of numerous hundred amino acids.
Already, the small proteins that were known to researchers are called peptides and function as signal molecules or hormones. They are developed when they split off from bigger precursor proteins.
“Our work now shows that peptides of a similar size can develop in a different way,” says Sandmann.
Also, such smallest-of-the-small proteins could bind very particularly to bigger proteins—but it remains indefinite if they could turn hormones or similar:
“We don’t yet know what most of these microproteins do in our body,” states Sandmann.
But the study does offer an inkling of what the molecules have the ability to do: “These initial findings open up numerous new research opportunities,” states van Heesch.
Certainly, the microproteins are much too significant for scientists to keep neglecting them. Van Heesch states the biomolecular and medical research communities are highly enthusiastic regarding such new findings.
One possible scenario would be “that these microproteins are involved in cardiovascular disease and cancer, and could therefore be used as new targets for diagnostics and therapies,” states Hübner. Several U.S.
Already, biotech companies are doing research in this direction. Also, the group behind the present study also has big plans: Their study analyzed 281 microproteins, but the goal currently is to extend the experiments to include many more of the 7,000 recently cataloged microproteins—in the belief that this will disclose several as-yet-unexplored functions.
Source:
Journal reference:
Sandmann, C-L., et al. (2023) Evolutionary origins and interactomes of human, young microproteins and small peptides translated from short open reading frames. Molecular Cell. https://doi.org/10.1016/j.molcel.2023.01.023