Chinese researchers have turned out to be successful in restoring the vision of mice with retinitis pigmentosa, which is considered to be one of the significant causes of blindness in humans.
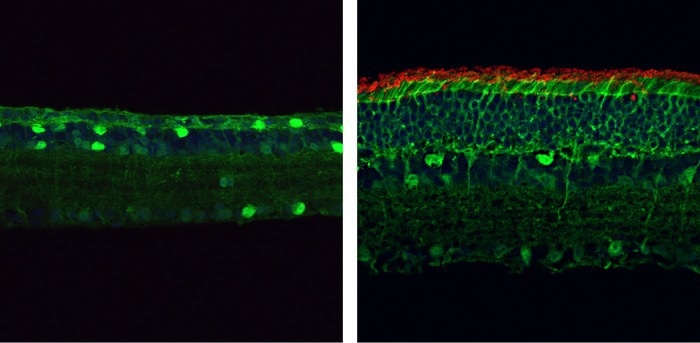 By the age of four months, the retinas of mice carrying a mutation in the gene encoding PDE6β (left) are thin and lack rod photoreceptors (red). But mice who have had this mutation corrected through the PESpRY system (right) have much thicker retinas containing numerous rod cells. Image Credit: © 2023 Qin et al. Originally published in the Journal of Experimental Medicine.
By the age of four months, the retinas of mice carrying a mutation in the gene encoding PDE6β (left) are thin and lack rod photoreceptors (red). But mice who have had this mutation corrected through the PESpRY system (right) have much thicker retinas containing numerous rod cells. Image Credit: © 2023 Qin et al. Originally published in the Journal of Experimental Medicine.
The study reported on March 17th, 2023, in the Journal of Experimental Medicine, makes use of a new and highly adaptable form of CRISPR-based genome editing with the ability to correct an extensive range of disease-causing genetic mutations.
Earlier, scientists have made use of genome editing to restore the vision of mice with genetic diseases, like Leber congenital amaurosis, that tends to impact the retinal pigment epithelium, known as a layer of non-neuronal cells in the eye that assists the light-sensing rod and cone photoreceptor cells. But the majority of the inherited forms of blindness, such as retinitis pigmentosa, are caused as a result of genetic defects in the neural photoreceptors themselves.
The ability to edit the genome of neural retinal cells, particularly unhealthy or dying photoreceptors, would provide much more convincing evidence for the potential applications of these genome-editing tools in treating diseases such as retinitis pigmentosa.”
Kai Yao, Professor, Wuhan University of Science and Technology
Retinitis pigmentosa could be caused by mutations in more than 100 different genes and is evaluated to impair the vision of 1 in 4,000 people. It starts with the death and dysfunction of dim light-sensing rod cells, before spreading to the cone cells needed for color vision, ultimately resulting in irreversible and severe vision loss.
Yao and colleagues have tried to rescue the vision of mice with retinitis pigmentosa which is caused by a mutation in the gene encoding a critical enzyme known as PDE6β. For this to be done, Yao’s team came up with a new and highly versatile CRISPR system known as PESpRY. It is possible to program to correct several different kinds of genetic mutation, irrespective of where they occur inside the genome.
While being programmed to target the mutant PDE6β gene, the PESpRY system had the potential to effectively correct the mutation and further restore the activity of the enzyme in the retinas of mice. This avoided the death of cone and rod photoreceptors and restored their normal electrical responses to light.
Yao and colleagues executed a range of behavioral tests to verify that the gene-edited mice maintained their vision even into old age. For instance, the animals were capable of finding their way out of a visually guided water maze nearly as well as normal, healthy mice and displayed normal head movements in response to visual stimuli.
Yao warns that much work still requires to be done to fix both the efficacy and safety of the PESpRY system in humans.
However, our study provides substantial evidence for the in vivo applicability of this new genome-editing strategy and its potential in diverse research and therapeutic contexts, in particular for inherited retinal diseases such as retinitis pigmentosa.”
Kai Yao, Professor, Wuhan University of Science and Technology
Source:
Journal reference:
Qin, H., et al. (2023) Vision rescue via unconstrained in vivo prime editing in degenerating neural retinas. Journal of Experimental Medicine. https://doi.org/10.1084/jem.20220776.