Infection risk is always there in those with compromised immune systems. Pseudomonas aeruginosa, a typical environmental bacterium, can colonize several body areas, including the lungs. This can result in persistent, chronic infections that can last a lifetime, a common occurrence in people with cystic fibrosis.
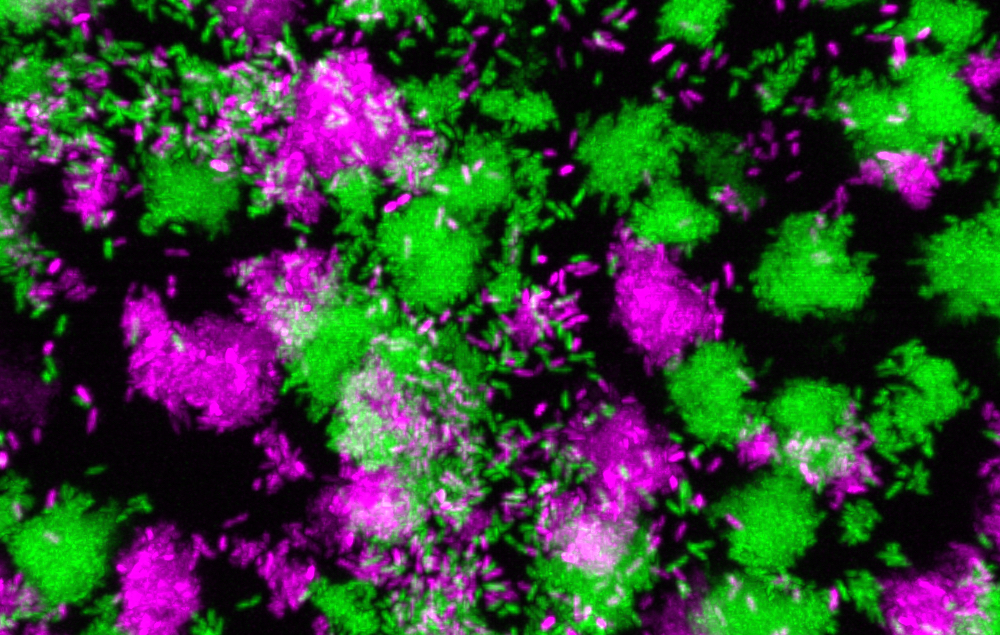 Pseudomonas aeruginosa clumps grown in synthetic cystic fibrosis sputum. Image Credit: Georgia Institute of Technology
Pseudomonas aeruginosa clumps grown in synthetic cystic fibrosis sputum. Image Credit: Georgia Institute of Technology
However, the bacteria occasionally alter their behavior and go into the bloodstream, turning persistent, localized infections into acute and possibly lethal infections. Although the flip has been studied extensively in lab settings over the years, it is still unclear how and why it occurs in humans.
However, Georgia Institute of Technology researchers have discovered the main mechanism underlying the change from chronic to acute P. aeruginosa infections.
A switch-operating gene was found by Marvin Whiteley, a professor in the School of Biological Sciences and the Bennie H. and Nelson D. Abell Chair in Molecular and Cellular Biology, and Pengbo Cao, a postdoctoral researcher in Whiteley’s group.
The researchers discovered a biomarker for the transition by examining the expression of bacterial genes in samples of human tissue.
Their study’s findings, which were published in the journal Nature, could assist in creating new therapies for acute infections that are fatal.
According to Whiteley and Cao, bacteria exhibit a variety of behaviors based on their environment, just like animals do. If the environment in the body changes, bacteria could act differently than they would in the absence of a chronic infection. This can result in an acute infection or sepsis, which must be treated immediately.
For years, people have been studying these bacteria in well-controlled lab environments, even though the lab is a place most microbes have never seen. Our study took a novel approach to look directly into the bacterium’s behavior in the human host.
Marvin Whiteley, Professor, School of Biological Sciences, Georgia Institute of Technology
The study team decided to examine samples of human tissue from bacterial lung and wound infections. Whiteley and Cao evaluated the abundance of each type of mRNA in the bacterium using genetic sequencing technology.
Inferring the behavior of a bacterium from its mRNA level is possible since the proteins that carry out all of the work in a cell are encoded by mRNAs.
Whiteley and Cao discovered that PA1414, one of the approximately 6,000 genes in P. aeruginosa, was more highly expressed in human tissue samples than all the other thousands of genes put together. The levels were so high that initially, Cao and Whiteley believed the PA1414 mRNA quantity might be an artifact—a problem with the sequencing techniques.
This particular gene is not expressed in the standard lab environment very much, so it was striking to see these levels. And at this point, the function of the gene was unknown.
Pengbo Cao, Postdoctoral Researcher, Georgia Institute of Technology
The lack of oxygen, according to the researchers, is what promotes the gene's high expression. Since bacteria typically experience oxygen deprivation during persistent infections, this is a prevalent environmental component of bacterial diseases. Further studies revealed that the gene controls bacterial respiration in low-oxygen environments.
Interestingly, the researchers discovered that the gene encodes a small RNA essential for bacterial respiration rather than a protein. The small RNA was given the designation SicX (sRNA inducer of chronic infection X).
The gene’s capabilities were then examined by the researchers using several animal infection models. They saw that the bacteria rapidly spread from chronic infections throughout the body when SicX was not present, resulting in systemic infection.
The researchers were able to conclude from the comparison that the gene is crucial for encouraging chronic localized infection. SicX could act as a biomarker for the move from chronic to acute infection, according to studies that also revealed that the expression of SicX instantly dropped during the shift from chronic to acute infection.
Whiteley added, “In other words, without the small RNA, the bacteria become restless and go looking for oxygen, because they need to breathe like we need to breathe. hat need causes the bacteria to enter the bloodstream. Now, we know that oxygen levels are regulating this transition.”
Treatments would undergo a paradigm shift if there were a better indicator of when infection would enter the bloodstream.
“If you can predict when an acute infection will occur, a patient could take a diagnostic test at home to determine if and when they may need to get treatment—before the infection becomes life-threatening,” Whiteley further stated.
The research offers solutions to the age-old concerns of how and why chronic illnesses become acute. The findings of the researchers also present prospects for the development of therapies that focus on this particular molecular behavior related to P. aeruginosa infections.
Cao concluded, “The chronic Pseudomonas infection is usually highly resistant to first-line antibiotics. By targeting this small RNA, we could potentially change the lifestyle of the bacteria to make it more susceptible to antibiotic treatments and achieve greater clearance of these dangerous infections.”
Source:
Journal reference:
Cao, P., et al. (2023). A Pseudomonas aeruginosa small RNA regulates chronic and acute infection. Nature. doi.org/10.1038/s41586-023-06111-7