Reviewed by Danielle Ellis, B.Sc.Nov 30 2023
The concept of irreversible inhibitors binding permanently to a target protein has garnered growing interest for potential applications in drug development.
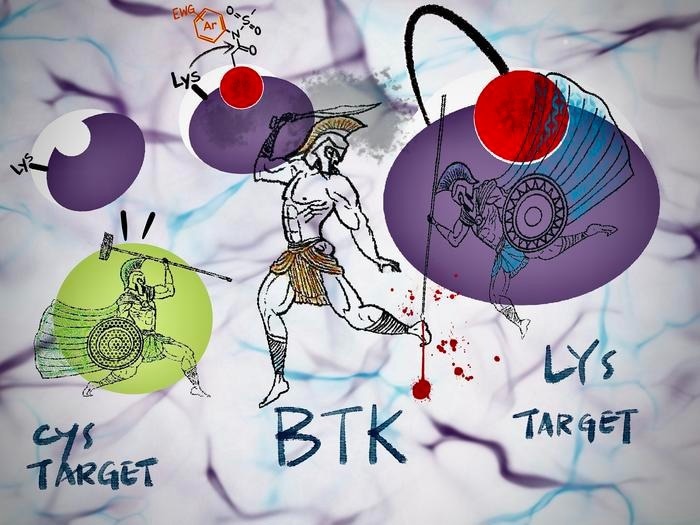 Lysine target engages with an irreversible inhibitor. The reactive ArNASA group chemically reacts with the protein’s amino acids to form a covalent bond, irreversibly inhibiting protein activity. Image Credit: KyotoU/Jake Tobiyama/Tomonori Tamura.
Lysine target engages with an irreversible inhibitor. The reactive ArNASA group chemically reacts with the protein’s amino acids to form a covalent bond, irreversibly inhibiting protein activity. Image Credit: KyotoU/Jake Tobiyama/Tomonori Tamura.
However, a significant challenge lies in the potential for protein mutations to render otherwise effective drugs pharmacologically inactive.
Traditional covalent inhibitors utilize reactive groups that induce a single reaction in target proteins, permanently deactivating them. However, mutations can occur more readily with certain amino acids, disrupting this deactivation process.
In response to this challenge, a team of researchers at Kyoto University has introduced a novel reactant that demonstrates effectiveness on proteins harboring drug-resistant mutations.
In Bruton-type tyrosine kinase (BTK), an important drug target, a mutation involving amino acids cysteine to serine—called C481S—is known, but we have not yet seen any for our lysine target. Still, it is significant that our irreversible inhibitor can at least address the C481S problem.”
Tomonori Tamura, Graduate School of Engineering, Kyoto University
Traditional irreversible inhibitors commonly employed in clinical settings exclusively interact with protein cysteine residues.
Moreover, cysteine, recognized as the most reactive among the 20 amino acids, is not plentiful at reaction or binding sites. Mutations can also transform cysteine into a different amino acid, rendering the cysteine-targeting irreversible inhibitors ineffective against drug-resistant proteins.
In contrast, N-acyl-N-arylsulfonamide, abbreviated as ArNASA, exhibits the ability to react with lysine residues and maintains high stability in serum-containing media and various physiological environments.
Utilizing this reaction property, we developed the first irreversible inhibitor of BTK, which has drug-resistant mutations.”
Tomonori Tamura, Graduate School of Engineering, Kyoto University
The Tamura team’s quest for valuable reactive groups may find success with ArNASA. Crucially, its electrophiles overcome limitations by minimizing hydrolytic inactivation and unintended interactions with off-target proteins.
Upon engagement with the irreversible inhibitor, the reactive group chemically reacts with the protein's amino acids, establishing a covalent bond. This results in an enduring binding site, effectively and irreversibly inhibiting protein activity.
Building upon an earlier NASA group, which was comparable in efficacy to ArNASA but ineffective in serum-containing media, Tamura’s team synthesized the new reactive group using aromatic amines as starting materials. The researchers then applied the ArNASA group to BTK, a crucial therapeutic target for blood cancers like chronic lymphocytic leukemia.
Our study will extend beyond cell-based research to in vivo, paving the way for developing drugs with diverse reactant groups acting on specific amino acids.”
Tomonori Tamura, Graduate School of Engineering, Kyoto University
Source:
Journal reference:
Kawano, M., et al. (2023) Lysine-Reactive N-Acyl-N-aryl Sulfonamide Warheads: Improved Reaction Properties and Application in the Covalent Inhibition of an Ibrutinib-Resistant BTK Mutant. Journal of the American Chemical Society. doi.org/10.1021/jacs.3c08740.