Research by Stony Brook Medicine nephrology specialists may help prevent or reduce diabetic kidney disease progression by targeting cellular signaling between kidney cells and inducing a specific gene. Diabetic kidney disease is the leading cause of chronic kidney disease worldwide.
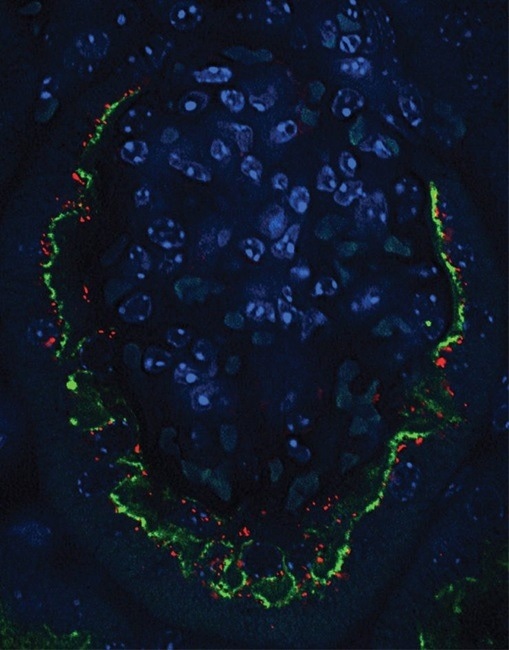 Shown from the study is an immunofluorescence image of the proximal tubule (in green), surrounding the glomeruli in the kidney with the activated calcium/calmodulin-dependent protein kinase 1 (CaMK1D), in red. Blue indicates cells in the kidney. Image Credit: Sandeep K. Mallipattu and Nehaben A. Gujarati
Shown from the study is an immunofluorescence image of the proximal tubule (in green), surrounding the glomeruli in the kidney with the activated calcium/calmodulin-dependent protein kinase 1 (CaMK1D), in red. Blue indicates cells in the kidney. Image Credit: Sandeep K. Mallipattu and Nehaben A. Gujarati
The National Kidney Foundation reports that over 35 million adults in the United States have kidney disease, which affects approximately one in seven adults. Additionally, one-third of Americans with diabetes also have kidney disease.
Researchers in nephrology have yet to develop a therapeutic approach to halt or slow the progression of diabetic kidney disease (DKD). A study by Nehaben A. Gujarati, PhD, and Sandeep K. Mallipattu, MD, of the Division of Nephrology and Hypertension at Stony Brook University, presents a murine model that may lead to a more effective treatment for DKD. The study's findings are published in the journal Nature Communications.
They employed a multi-omics approach, showing that inducing the human KLF6 transcription factor and modulating cell signaling between podocytes and proximal tubule cells reduces podocyte loss, proximal tubule dysfunction, and subsequent interstitial fibrosis that occurs in later disease stages.
Podocytes are crucial for regulating glomerular function, which involves filtering waste and excess water from the blood to form urine. Proximal tubule cells, the primary cell type in the kidney, perform various functions, including reabsorbing water, glucose, and proteins from the glomerular filtrate and maintaining electrolyte balance and fluid equilibrium. Both cell types are essential for preventing DKD progression.
Drs. Mallipattu and Gujarati showed in this DKD model that KLF6 initiates Apolipoprotein J (ApoJ) release from podocytes, which then activates CaMK1D in proximal tubule cells, preventing mitochondrial damage and stopping DKD progression.
This cell to cell communication through this signaling mechanism in the kidney might serve as a protective mechanism in the early stages of DKD.”
Dr. Sandeep K. Mallipattu, Senior Author and DCI-Leibowitz Professor, Stony Brook University
This study utilized murine models, human cells, and kidney tissue from human biopsies at different stages of DKD.
In combination, findings from these studies highlight that targeting podocyte-proximal tubule signaling by enhancing Apolipoprotein J-CaMK1D could prove to be a therapeutic strategy in slowing down or perhaps event preventing DKD.”
Dr. Sandeep K. Mallipattu, Senior Author and DCI-Leibowitz Professor, Stony Brook University
Drs. Gujarati and Mallipattu will continue investigating this approach for treating DKD. Their next step involves assessing whether activating the relevant signaling pathway in the kidney through pharmacological interventions can prevent DKD in individuals with diabetes.
Source:
Journal reference:
Gujarati, N. A., et al. (2024) Podocyte-specific KLF6 primes proximal tubule CaMK1D signaling to attenuate diabetic kidney disease. Nature Communications. doi.org/10.1038/s41467-024-52306-5.