The mechanisms behind intellectual disabilities and autism remain largely unknown. However, researchers from the labs of Prof. Pierre Vanderhaeghen and Prof. Vincent Bonin at the VIB-KU Leuven Center for Brain & Disease Research and NERF have identified that mutations in the SYNGAP1 gene interfere with the extended development of human neurons, a process believed to be critical for normal cognitive function.
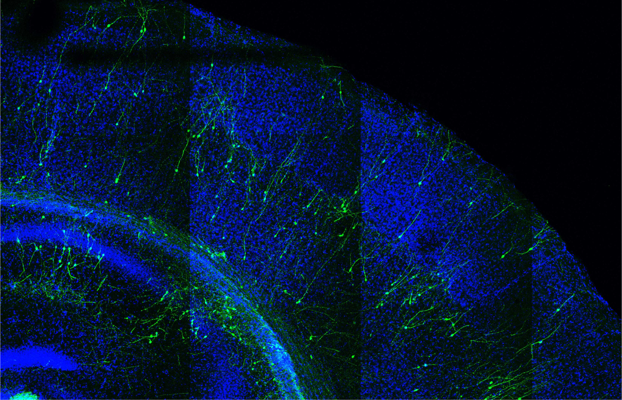 Mouse cortex (in blue) transplanted with human neurons (in green). Image Credit: VIB-KU Leuven
Mouse cortex (in blue) transplanted with human neurons (in green). Image Credit: VIB-KU Leuven
Their research, which was published in the journal Neuron, has intriguing ramifications for the comprehension and advancement of treatments for autism and intellectual disabilities.
Among mammals, the human brain stands out for its unusually protracted growth. The human brain's neurons, especially those in the cerebral cortex, the main location for cognitive processes take years to fully grow, in contrast to those in other species.
Neoteny is the term for this process, which is believed to be essential for the development of some of the more complex cognitive abilities that define species. Disturbances in this extended development may be the cause of autism and certain types of intellectual disability. This theory had never before been investigated in human neurons.
A Window into the Maturing Brain
According to earlier research, one of the main causes of these disorders is mutations in this gene, but nothing was known about the precise consequences of its disruption on human brain neurons.
The absence of trustworthy experimental techniques to monitor human cortical neuron growth in a living brain was a significant barrier to research into developmental disorders of the human brain until recently.
SYNGAP1 is essential for the extended developmental timeline of human cortical neurons, according to research from the VIB-KU Leuven Center for Brain & Disease Research and NERF (Neuro-Electronics Research Flanders, supported by imec, KU Leuven, and VIB). This proves a connection between autism, intellectual handicap, and accelerated neural growth.
Using a xenotransplantation model, the researchers grafted human neurons with the SYNGAP1 mutation into mice's brains and then examined the development and function of the neurons to learn more about how the mutation impacts human neuron development in vivo.
Faster is Not Better
The researchers investigated the effects of the SYNGAP1 mutation by studying transplanted human neurons in the mouse brain, focusing on the circuit level—examining the connections between neurons that serve specific functions in the brain.
We saw that the SYNGAP1 mutant neurons looked normal in most aspects, but that they displayed a strong acceleration of their development. Most strikingly, they connected much faster with other neurons."
Dr. Ben Vermaercke, Study First Author, VIB-KU Leuven Center for Brain & Disease Research
Dr. Vermaercke and his colleagues specifically found that the impaired neurons responded to visual stimuli months before the typical developmental schedule and integrated into cortical circuits more quickly, suggesting that the neurons' faster maturation resulted in precocious functionality within brain circuits.
This accelerated development of SYNGAP1 mutant neurons could alter the early function and plasticity of infant brain circuits, although this needs to be studied further by experimental and clinical investigations. The important role of neoteny for normal human brain development highlights how its disruption can lead to neurodevelopmental diseases.”
Pierre Vanderhaeghen, Professor, VIB-KU Leuven Center for Brain & Disease Research
Vanderhaeghen added, “Early defects in the development of human cortical neurons could have important implications for the diagnosis and treatments of the patients affected by SYNGAP1, and potentially in patients presenting other forms of intellectual disability or autism.”
The transplantation model we developed enables, for the first time, the study of human neuronal diseases in vivo at both the functional and circuit levels. This breakthrough constitutes a promising model for understanding neurological diseases and testing new treatments.”
Vincent Bonin, Professor, VIB-KU Leuven Center for Brain & Disease Research
Source:
Journal reference:
Vermaercke, B., et al. (2024) SYNGAP1 deficiency disrupts synaptic neoteny in xenotransplanted human cortical neurons in vivo. Neuron. doi.org/10.1016/j.neuron.2024.07.007.