Reviewed by Danielle Ellis, B.Sc.Nov 29 2024
Natural killer (NK) cells play an important role in the innate immune response to cancer and viral infections, and their presence in tumors correlates with improved patient outcomes in a variety of cancers. However, NK cells in the tumor microenvironment frequently become functionally tired, with reduced numbers and compromised functions as a result of immune regulatory signal imbalances.
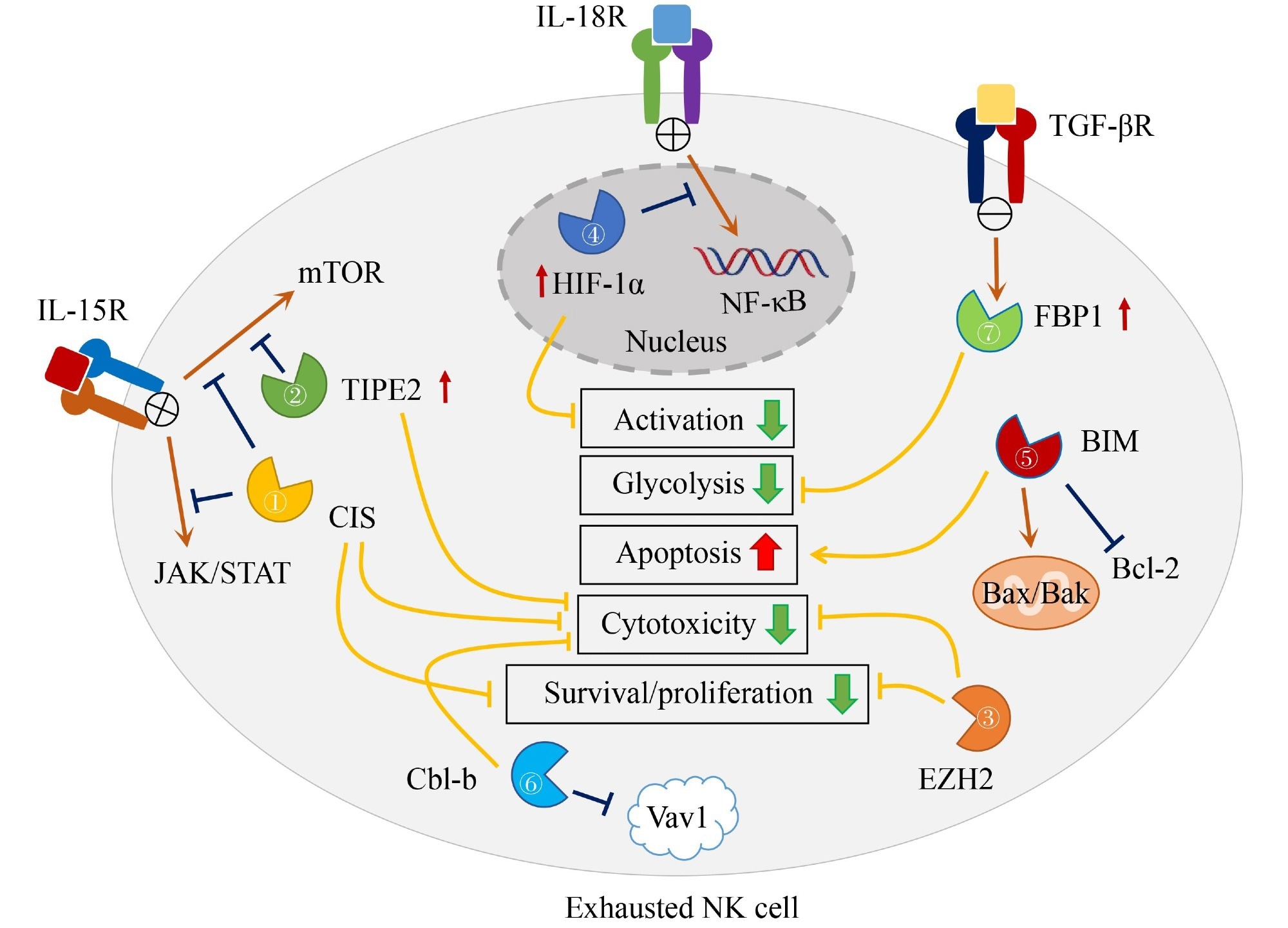
Image Credit: Frontiers Journals
Immune checkpoint receptors, which are inhibitory cell surface receptors that have the ability to block antitumor immunity, have an impact on this exhaustion. NK cells’ metabolism, proliferation, survival, and cytotoxic function are all impacted by intracellular checkpoint molecules such as FBP-1, EZH2, CIS, TIPE2, and HIF-1α, which significantly contribute to NK cell exhaustion. Since these compounds have a consistent effect on antitumor immunity in various circumstances, they provide a universal target for cancer immunotherapy.
The presence of HLA class I molecules, which interact with inhibitory KIRs on NK cells to limit their activity, further complicates the complex interaction between NK cells and malignancy. The interactions between NK cells and tumor cells are made more complex by the polymorphism of KIRs and their ligands.
Furthermore, several inhibitory receptors that are frequently expressed in tumor tissues, such as PD-1, TIGIT, TIM-3, and CD94/NKG2A, act as context detectors, affecting antitumor immunity according to the presence and concentration of their ligands.
Intracellular checkpoint molecules, such as BIM, which regulates apoptosis in NK cells, can be targeted to improve antitumor responses. The lack of BIM in NK cells has been demonstrated to boost their resistance to apoptosis and accumulation in later stages of maturation, potentially improving their antitumor properties. Similarly, Cbl-b, an E3 ubiquitin protein ligase, inhibits NK cell cytolytic activity and thereby improves NK cell-mediated control of tumor metastasis.
IL-15 activates CIS, a negative regulator of IL-15 signaling in NK cells, and its absence increases NK cell sensitivity to IL-15, proliferation, survival, and cytotoxicity against tumor cells. EZH2, a component of the polycomb repressive complex 2, operates as a negative regulator of NK cell effector actions, and targeting it may aid in NK cell immunotherapy. FBP1, a gluconeogenesis enzyme, inhibits glycolysis in NK cells and can restore NK cell function, indicating a role in NK cell exhaustion.
IPE2, a negative regulator of IL-15 signaling, is increased under various conditions, inhibiting downstream AKT and Ral activation. The lack of TIPE2 in NK cells leads to increased functional maturation, cytotoxicity, and IFN-γ production, suggesting its potential as a checkpoint for NK cell immunotherapy. HIF-1α, a transcription factor involved in cellular responses to hypoxia, inhibits IL-18-driven NF-κB activation and antitumor activity of tumor-infiltrating NK cells. Inhibiting HIF-1α in NK cells may improve the treatment of solid tumors.
Since the effectiveness of checkpoint receptors on the cell surface is frequently tumor-specific and reliant on ligand interactions, the therapeutic potential of targeting intracellular checkpoint molecules in NK cells is more universal. To improve NK cell immunotherapy, targeting intracellular checkpoints could be used in conjunction with other tactics such as tumor sensors, immune checkpoint blockade, and synthetic gene circuits.
Finding targetable checkpoints requires an understanding of the mechanisms of action of emerging intracellular checkpoint molecules, which provide new targets for enhancing NK cell antitumor efficacy.
CRISPR/Cas9, shRNA, and small-molecule inhibitors are all strategies for targeting intracellular checkpoint molecules, but each has its own set of obstacles and limits. The advancement of NK cell genetic editing methods, as well as the upgrading of viral transfection platforms, are critical for stable gene overexpression in NK cells. The identification and targeting of these intracellular checkpoints show promise for improving the efficacy of NK cell immunotherapy in cancer treatment.
Source:
Journal reference:
Huang, Y., et al. (2024) Intracellular checkpoints for NK cell cancer immunotherapy. Frontiers of Medicine. doi.org/10.1007/s11684-024-1090-6