Reviewed by Danielle Ellis, B.Sc.Dec 4 2024
Millions of people worldwide suffer from Alzheimer's disease, a crippling illness that gradually robs people of their memory and cognitive abilities. Mutations in multiple genes, including APP, PSEN1, and PSEN2, are associated with familial Alzheimer's disease (FAD), a rare inherited form of the disease.
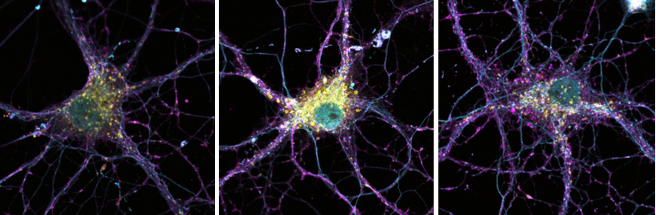 Hippocampal neurons with staining for endosomes (turquoise and purple) and lysosomes (yellow). When the PSEN2 gene is absent or carries a familial Alzheimer-related mutation (middle), the lysosomes become noticeably enlarged. Image Credit: VIB-KU Leuven Center for Brain & Disease Research
Hippocampal neurons with staining for endosomes (turquoise and purple) and lysosomes (yellow). When the PSEN2 gene is absent or carries a familial Alzheimer-related mutation (middle), the lysosomes become noticeably enlarged. Image Credit: VIB-KU Leuven Center for Brain & Disease Research
Little is known about the effects of PSEN2 mutations until now. A research team at VIB-KU Leuven, led by Professor Wim Annaert, has clarified how mutated PSEN2 speeds up the course of FAD. Their research, published in the journal Nature Communications, provided a fresh understanding of the processes underlying this hereditary type of illness.
The hallmarks of Alzheimer's disease include a build-up of amyloid plaques in the brain, behavioral abnormalities, and a progressive loss of cognitive function and memory. These plaques develop when the γ-secretase complex, among other things, “cuts into pieces” a protein known as APP (amyloid precursor protein).
These fragments include β-amyloid (Aβ) peptides, which can aggregate to create dangerous plaques. Different variants of the γ-secretase complex contain either PSEN1 or PSEN2, which affects where it is located within the cell and suggests that the two have slightly different functions.
FAD is an inherited form of Alzheimer's disease that usually manifests earlier than the more prevalent late-onset form. Symptoms can sometimes begin as early as a person's 30s or 40s and are caused by mutations in the genes encoding these functionally related proteins.
While it is well known that toxic Aβ fragments accumulate early in FAD and cause brain damage, it was unclear how exactly PSEN2 mutations speed up this process. To learn more about how the disease progresses in these FAD cases, Professor Wim Annaert's lab at the VIB-KU Leuven Center for Brain & Disease Research compared the effects of a mutant form of the PSEN2 gene with those of a loss of PSEN2.
A Double-Edged Knife
The researchers used mouse models that resembled Alzheimer's disease to examine the effects of PSEN2 loss and a mutation linked to FAD. Amyloid plaques accumulate in the brain more quickly in both the presence of mutated PSEN2 and its absence.
Furthermore, there were notable memory deficits in both the PSEN2-mutant and PSEN2-deficient mice. The hippocampus, a crucial region for working memory that is mainly impacted in AD patients, showed both structural and functional alterations in correlation with these impairments.
This part of the brain is crucial for tasks like following multi-step instructions, cooking recipes, or remembering directions while walking or driving.
The researchers consistently found that missing or impaired PSEN2 negatively impacted the function of synapses, the connections between brain cells that enable communication. Additionally, learning and memory were negatively impacted by the impairment of long-term potentiation, the process by which synapses become stronger over time.
The team looked into mechanisms within important brain cell neurons to determine the cause of these impairments. They discovered that PSEN2 is essential for preserving normal cellular functions in late endosomes and lysosomes, which are the cells' “garbage disposal system” for breaking down and recycling proteins.
These processes were disturbed by a lost or mutated PSEN2 gene, which resulted in a toxic accumulation of APP fragments, including Aβ peptides. Therefore, in addition to increasing the number of harmful amyloid plaques, PSEN2 gene impairment results in a degradative bottleneck inside nerve cells, which impairs the recycling of other molecules, including those that are in charge of signal transmission between nerve cells.
These results highlight the multi-faceted impact of PSEN2 mutations—on one hand, driving toxic amyloid accumulation, and on the other, impairing fundamental cellular maintenance systems, amplifying synaptic and cognitive decline.”
Anika Perdok, Study First Author, VIB-KU Leuven Center for Brain & Disease Research
What this Means for Alzheimer’s Research
Our findings underscore the importance of PSEN2 in regulating brain cell health. Targeting the underlying cellular dysfunction caused by PSEN2 mutations could be a potential route for Alzheimer’s treatments. To translate these insights into treatments, we require further research, but our work suggests that therapies aimed at restoring the function of endosomes and lysosomes or normalizing γ-secretase activity in these organelles could help mitigate the effects of PSEN2 mutations and slow disease progression in FAD.”
Wim Annaert, Professor, VIB-KU Leuven Center for Brain & Disease Research
Source:
Journal reference:
Perdok, A., et al. (2024) Altered expression of Presenilin2 impacts endolysosomal homeostasis and synapse function in Alzheimer’s disease-relevant brain circuits. Nature Communications. doi.org/10.1038/s41467-024-54777-y.