Attrition in the therapeutic pipeline can typically result from a lack of translational efficacy between the pre-clinical phase and the clinic. Organoids offer much promise in drug screening and disease modeling since they are good at resembling tissue structure and functionality and highlight more predictive responses to drugs. However, difficulties associated with practically adopting organoids, such as reproducibility, assay complexity, and being able to scale up, have yet to become widely adopted as a primary drug discovery screening method.
To ease the bottlenecks associated with labor-intensive manual protocols, the CellXpress.ai™Automated Cell Culture System has been developed. It is a powerhouse workstation that automates the whole organoid culture process for long-term complex workflows. The CellXpress.ai system offers media exchange, passaging, plating, organoid monitoring, and endpoint assay execution, as well as performing complex image analysis. Below are the results from the automation of several typically used organoid protocols, including the culture of 3D organoids in matrix domes.
For this study, healthy intestinal organoids were cultured, passaged, and expanded in Matrigel® domes (24-well). The organoids were cultured with automated media exchange and monitored every 24 hours through machine learning-assisted imaging. Following 5–6 days like this, the organoids were collected through automation, purified from Matrigel, dispersed, combined with fresh Matrigel, and re-plated.
The organoids self-organized and created complex crypt structures. They were observed in transmitted light, and machine learning-based image analysis was utilized to determine the organoid number, size (by area), and optical density. For the endpoint assay (96-well), the organoids were stained for viability markers and watched for time-dependent and concentration compound effects on healthy intestinal organoids (toxicity evaluation) or patient-derived colorectal cancer organoids (drug screening).
Cell culture processes that are automated and powered by imaging and machine learning-controlled decision-making could potentially bring 3D biology to the next level, increasing throughput and reproducibility for drug discovery and disease modeling applications.
Methods
Instrumentation
The CellXpress.ai is a powerful new cell culture system that automates the whole cell culture process with a liquid handler, integrated incubator, and image-based decision-making.
It is a hands-off automated solution that can manage the demands of feeding and passaging schedules through monitoring cell culture development with occasional imaging and analysis.
It also leverages machine learning to begin passaging, endpoint assay, or troubleshooting steps. Fluorescent (FL) images were obtained on the ImageXpress® Confocal HT.ai High-Content Imaging System (Molecular Devices) using the MetaXpress® High-Content Image Acquisition and Analysis Software. For intestinal organoids, Z-stack images were obtained with the 4x or 10x objectives using confocal mode. MetaXpress or IN Carta® Image Analysis Software was utilized in all analysis.
Cell Culture Protocols
A protocol for 3D intestinal organoids* was developed as a derivative of primary mouse intestinal cells through established methods (STEMCELL Technologies). The cells were cultured and differentiated using the STEMCELL Technologies protocol. IntestiCult™ Organoid Growth Medium (STEMCELL Technologies) was used for the cell culture. The cells were seeded in 50 % growth-factor reduced Matrigel or Cultrex (Corning) domes in a 24-well plate format and fed fresh media every second day for 7–10 days. The Intestinal organoids were subsequently passaged- dissociated and re-plated into fresh Matrigel domes.

Figure 1. The CellXpress.ai cell culture system components and functionality. Image Credit: Molecular Devices UK Ltd
Organoid Cell Culture Automation
Organoid culture in Matrigel domes was completed in accordance with the basic STEMCELL Technologies protocol recommended for mouse intestinal organoids. The organoids were raised and passaged in 24-well plate format using a single dome 40–50 μl, 50–60 % Matrigel or Cultrex.
The organoid plating began with an organoid suspension in Matrigel, which was transferred into a 96-well deep well block that had been pre-chilled. Four pipette tips were used to pipette the suspension into a 24-well plate, four tips at a time. A test involving plating into a 96-well format also occurred. The organoid's feeding was completed by removing the media and adding fresh media, four wells at a time.
Imaging and monitoring the organoid was completed in transmitted light utilizing the CellXpress.ai automated cell culture system and 2x or 4x magnification. A machine learning-based protocol was used for image analysis. The analysis examined organoid number, total area and mean, density, and other measurements.
Passaging organoids were established every four days, as directed by the user, or dependent on automatic decision-making based on one or more chosen measurements (e.g., organoid density or total area). The organoid passaging was completed through a combination of external centrifugation steps and pipetting steps to optimize the workflow of the mouse organoids. Modifications to the flow rates, pipetting steps, repeats, centrifugation speed, etc., can be customized by altering the appropriate “fine-tuning” steps.
Regarding the passaging process, the media was removed, and the Matrigel domes were incubated using a Gentle cell dissociation reagent, followed by rigorous pipetting to break the Matrigel domes. The mixture was harvested into a 96-deep-well block.
Optional pooling of two wells into one was completed, and then centrifugation on an external centrifuge for five minutes, set to a speed of 400 g. The block was then replaced, the media removed, and the organoids were washed once. Following a second centrifugation, most of the supernatant was aspirated, and the organoids were broken through rigorous pipetting with smaller tips. Fresh Matrigel was then added to the correct volume, combined, and seeded into a new plate.
The organoid was stained/imaged for the endpoint assay through FL imaging with the ImageXpress Confocal HT.ai instrument.
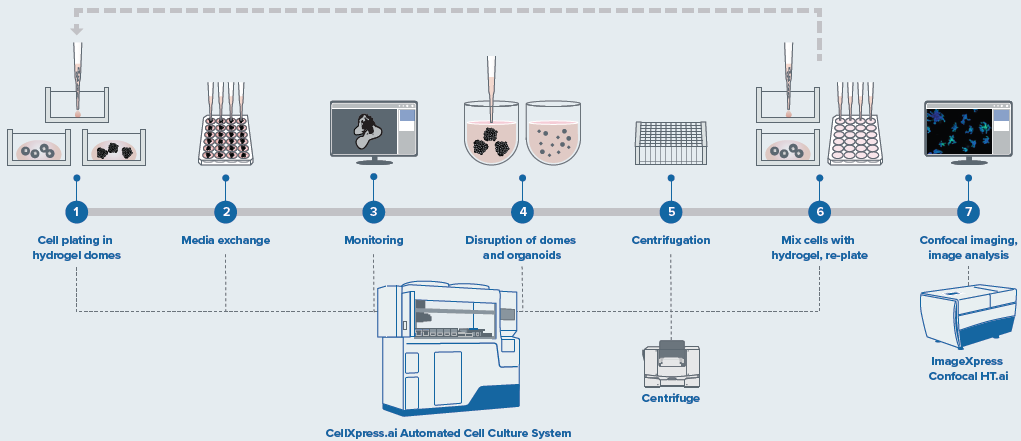
Figure 2. Schematic diagram of automated organoid culture and passaging protocol. Image Credit: Molecular Devices UK Ltd
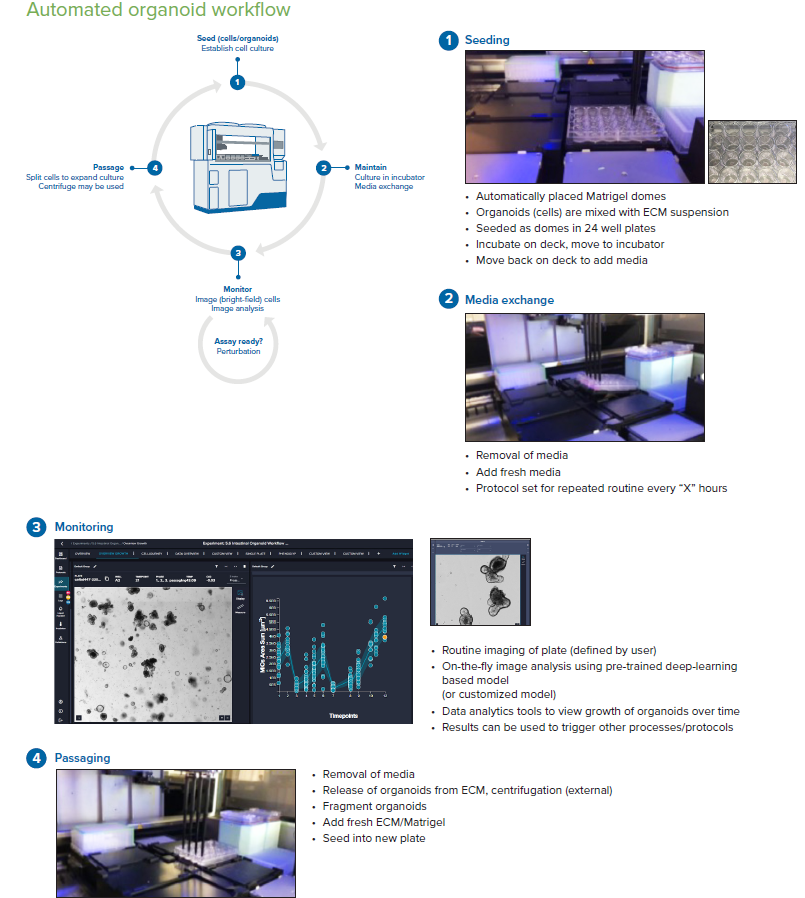
Figure 3. Steps of the organoid culture and passaging protocol. Image Credit: Molecular Devices UK Ltd
The workflow steps, including seeding, monitoring, media exchange, and passaging of the intestinal organoids, are programmed through pre-set protocol “phases”. The first phase is seeding, which involves placing Matrigel organoids into 24-well plates. The second phase involves feeding/monitoring/passaging steps with parameters and timing defined by the user.
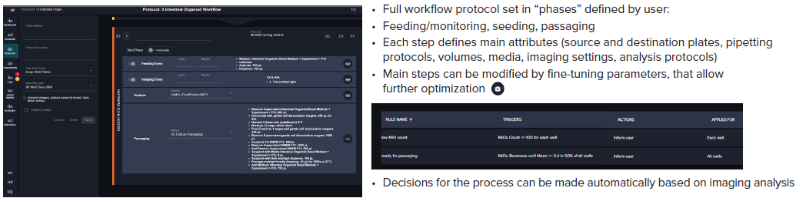
Figure 4. Steps of the organoid culture and passaging software protocol. Image Credit: Molecular Devices UK Ltd
Results
A continual culture of intestinal organoids has been performed for over a month. The automated seeding of the organoid domes enables consistent size and accurate centering of the domes in 24-well plates, making imaging and image analysis easier and more accurate. The organoids grow as anticipated throughout automated culture, forming protrusions consistent with common intestinal organoid morphology.
The distribution and number of organoids proved to be consistent across the wells. Daily images were taken using a 2x objective, and the image analysis was completed using a pre-trained machine-learning model. The image analysis enabled the finding of organoids and the determination of the number of organoids, total and mean area, organoid density, granularity, roundness, and other morphological criteria.
The software enables a review of the organoid domes throughout the culture and also offers on-the-fly analysis and time course plots that represent a variety of measurements. Exporting the data into Excel datasheets for further analysis is also possible. Figure 6 indicates alterations in the organoid's area sum and the number of organoids over time.
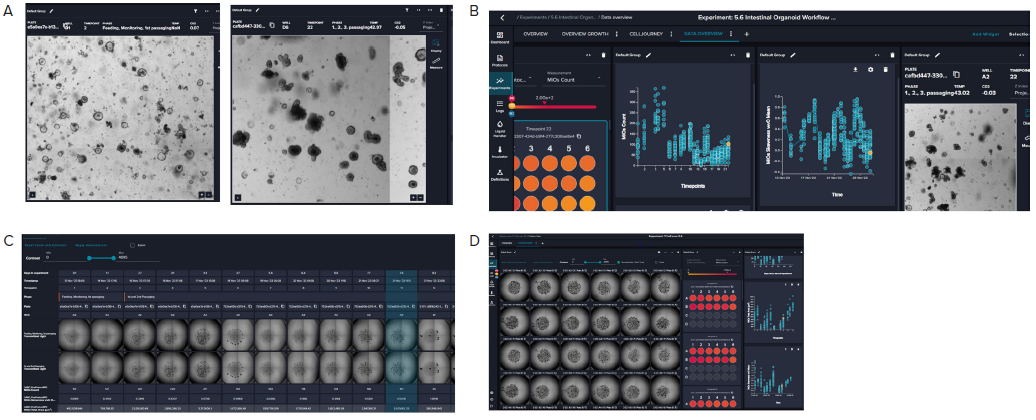
Figure 5. A. Representative images (4X) of organoid culture taken at different time points of the continuous culture. B. Graphical representation
of organoid analysis: organoid count and skewness over time. Skewness includes a combination of optical density, granularity, and other optical
parameters. The value is useful in defining the time for organoid passaging. C. Imaging of a single well over time showing the “cell journey”. D. Tiled 4X
images of organoid domes from a 24-well plate. Image Credit: Molecular Devices UK Ltd
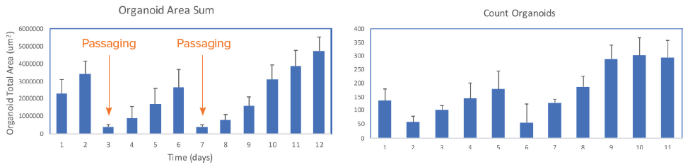
Figure 6. Presentation of organoid count and organoid total area (area sum) over time. Averages and STDEV were calculated from 24 wells of the plate. Image Credit. Molecular Devices UK Ltd
Summary
- Organoid technologies are seen as game-changing in disease modeling and drug screening. They resemble tissue structure and functionality better and indicate more predictive responses to drugs.
- Issues associated with the practical adoption of organoids, like assay complexity, lack of reproducibility, and scaling ability in terms of screening, have reduced more ready adoption as a primary screening method in drug discovery.
- This study demonstrated how researchers can eliminate the bottlenecks resulting from labor-intensive, complex protocols to improve drug screening productivity.
- The CellXpress.ai Automated Cell Culture System is a powerful new solution that enables labs to automate the whole cell culture process—including assay set-up, screening, and data analysis—with machine learning-powered workflows that make assays more reproducible and reliable.
- Automated cell culture processes powered by machine learning-controlled decision-making and imaging can move 3D biology to the next level by improving throughput and reproducibility and allowing a range of high-throughput drug discovery and precision medicine applications.
About Molecular Devices UK Ltd
Molecular Devices is one of the world’s leading providers of high-performance life science technology. We make advanced scientific discovery possible for academia, pharma, and biotech customers with platforms for high-throughput screening, genomic and cellular analysis, colony selection and microplate detection. From cancer to COVID-19, we've contributed to scientific breakthroughs described in over 230,000 peer-reviewed publications.
Over 160,000 of our innovative solutions are incorporated into laboratories worldwide, enabling scientists to improve productivity and effectiveness – ultimately accelerating research and the development of new therapeutics. Molecular Devices is headquartered in Silicon Valley, Calif., with best-in-class teams around the globe. Over 1,000 associates are guided by our diverse leadership team and female president that prioritize a culture of collaboration, engagement, diversity, and inclusion.
To learn more about how Molecular Devices helps fast-track scientific discovery, visit www.moleculardevices.com.
Sponsored Content Policy: AZO Life Sciences publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of AZO Life Sciences which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.