Discovering effective anti-cancer drugs and drug combinations is crucial for therapy success. There is a critical need to create methods to efficiently test drug efficacy and discover new viable therapeutic targets. 3D cancer cell models, like tumoroids, are tools of great value for cancer research and drug development because they are highly representative of the structure and behavior of tumors. Manual 3D cell culture workflows are not time efficient, prone to error, and inconsistent, making their adoption for high-throughput drug screening challenging.
To standardize and expedite the spheroid assay, Molecular Devices has created 3D cell culture automation methods that use the CellXpress.ai™ Automated Cell Culture System to offer automated passaging, plating, media exchange, and organoid monitoring in response to compound treatment, and endpoint assays. This study describes the automation of a colorectal cell culture workflow, where the culture was automated and imaging of colorectal cancer 3D spheroids derived from HCT116 cell lines in U-shape low attachment plates.
HCT116 cells were expanded in 2D and spheroids formed following the automated dispensing of the cell suspension into U-shape 96 or 384-well plates. Forty-eight hours were allowed to elapse, and then the spheroids were treated with several anti-cancer compounds at several concentrations for 3–5 days, followed by imaging and staining. Cell plating, media exchange, compound addition, and staining were completed automatically by the CellXpress.ai system. Throughout cell culture automation, spheroids were monitored using transmitted light to analyze phenotypic transformations, including the inhibition of growth and disintegration of spheroids.
The spheroids were stained with a combination of viability dyes Calcein AM and EtHD and the Hoechst nuclear stain for the endpoint assay. Subsequently, the spheroids were imaged and examined for spheroid size and live-dead cell scoring. Furthermore, the ATP content was measured utilizing a CellTiter-Glo assay. Luminescent read-outs were gained with the SpectraMax® iD3 Multi-Mode Microplate Reader. A concentration-dependent decrease in ATP content was observed, along with inhibition of spheroid growth and cell death in response to anti-cancer compounds.
Methods
Automation of Organoid Workflow
The new CellXpress.ai cell culture system automates the cell culture process with a liquid handler, integrated incubator, and image-based decision-making. This hands-off solution manages demanding passaging and feeding schedules by monitoring cell culture development with periodic imaging and examination. It also uses machine learning to start the next processing step or troubleshoot problems.
Cell Culture Protocols
3D spheroids were derived from HCT116 colorectal cells (ATCC) using U-shaped ultra-low attachment 96-well plates (Greiner). The steps for cell culture included seeding, media exchange, compound addition and stainings. Imaging and analysis were completed using the CellXpress.ai system. Spheroid imaging was completed throughout culture in transmitted light (TL) using 4X magnification. The endpoint assay imaging was completed with 10x magnification utilizing fluorescent imaging (FL) and analysis for organoid size. The intensities of viability markers were based on staining with EtHD, Calcein AM, and Hoechst nuclear stain. Analysis in TL was completed through the IN Carta® Image Analysis Software’s SINAP model and the FL analysis was completed via CME analysis protocol.

Figure 1. The CellXpress.ai cell culture system components and functionality. Image Credit: Molecular Devices UK Ltd
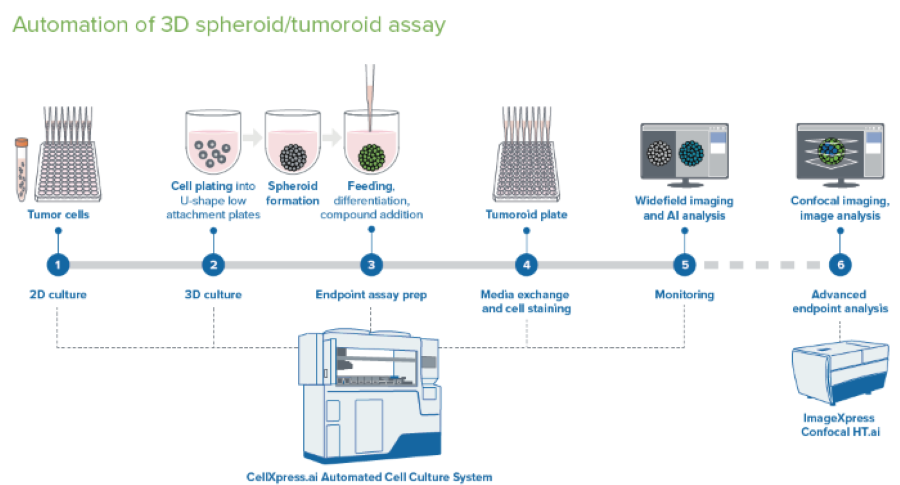
Figure 2. Schematic diagram of automated spheroid culture. Image Credit: Molecular Devices UK Ltd
Brief Description of Spheroid Protocol
The protocol was initiated from media with cell suspension deposited into a deep well reservoir (a 12-well reservoir was used here). Cell suspension was then added to the 96-well U-bottom low attachment plates (Greiner or Corning).
Plates were moved into the incubator, and incubation continued while imaging every 12 or 24 hours with 4x or 10x magnification in TL. Detecting spheroids and evaluating size and density through image analysis was completed on the fly.
Compound Addition: 48 hours after plating, 50 μl of media was discarded, and 2x concentrations of compounds at 50 μl volumes were added utilizing the “different media” protocol. The compounds were pre-diluted in the 96-well deep well block and subsequently added from a single-column block into four columns of the 96-well plate. The compounds were each added as a step that needed a plate map definition for each compound, triggering a compound addition step.
Staining Spheroids: The organoids were stained with a premixed solution of three viability dyes after three days. The staining was completed as a media exchange step. To eliminate cross-contamination, the tips must be discarded and not re-used. The staining incubation was completed in the incubator for one hour. A single washing step was completed with the media exchange (feeding) phase with PBS.
Imaging of the spheroids was completed using a pre-defined protocol with DAPI, FITC, and TexasRed. 10–15 steps, 10–15 μm apart, with offset 50–100, Z-stacks around focus. The analysis used the best-focus projections. CME analysis protocol in IN Carta® Image Analysis Software was utilized in defining the spheroids intensities and sizes with various channels.
Transformations in the spheroid area and average intensities, as well as the ratios of live/dead average intensities were utilized in evaluating the compound effects. To arrive at a phenotypic characterization of cells with more detail, it is recommended to use the ImageXpress® Micro Confocal system.
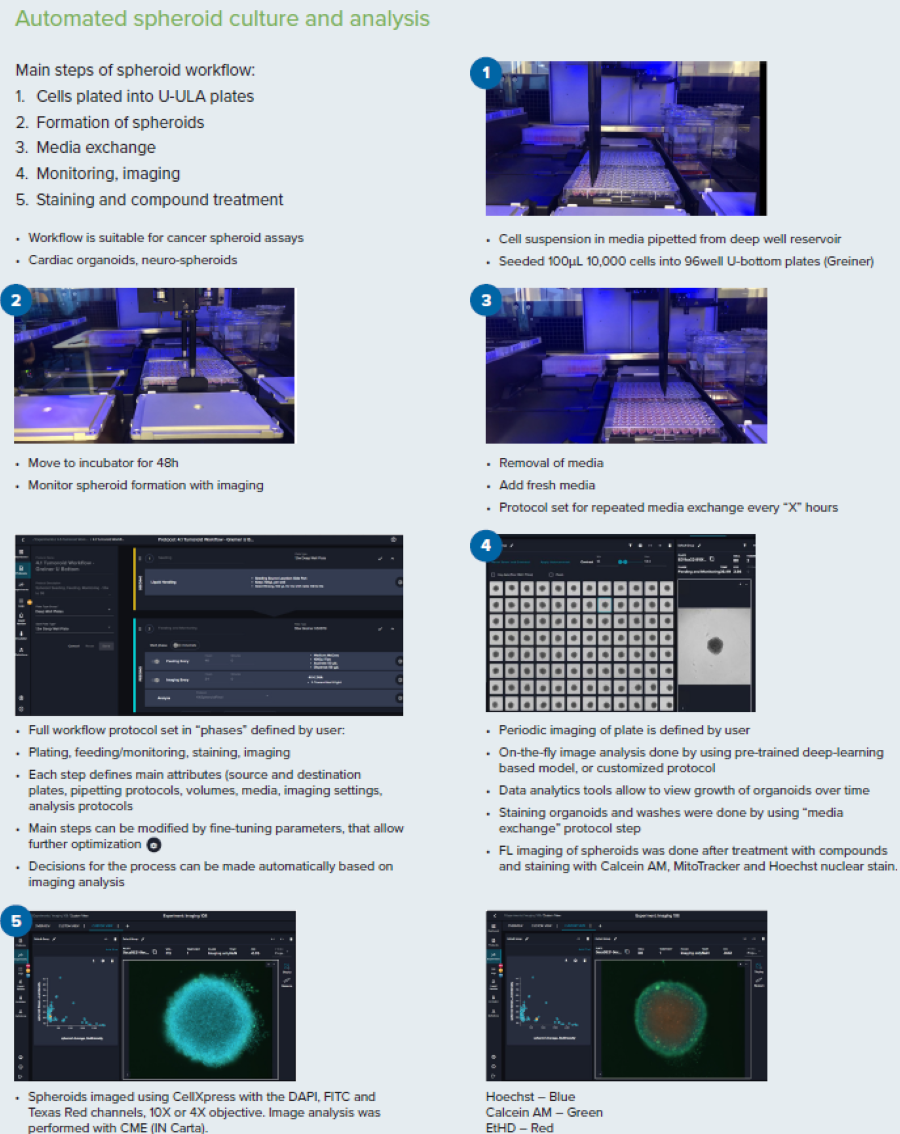
Figure 3. Steps for the spheroid culture and analysis workflow. Image Credit: Molecular Devices UK Ltd
Results
Phenotypic Evaluation of Compound Effects
The workflow that allows for the formation of spheroids/tumoroids is demonstrated here, using the example of HCT116 colorectal carcinoma cells. The spheroids were developed and maintained with the automated culture by CellXpress.ai instrument. Following three days of compound treatment with anti-cancer compounds (doxorubicin, staurosporine, paclitaxel), the spheroids were stained using cell viability dyes EtHD (dead cells) and Calcein AM (live cells), and nuclear stain Hoechst. The spheroids were imaged through the use of appropriate fluorescent channels DAPI, FITC and Texas Red, making use of the best focus projection images from Z-track of images taken at 10x magnification.
An evaluation of the fluorescence intensities and the live/dead staining ratios was completed. Figure 5 indicates a decrease in the live/dead marker intensities ratios with increased compound concentrations. In addition, CellTiter Glow reagent (a measure of ATP content) observed a lowering of cell viability. A decrease in ATP content was also seen with the increase in compound concentrations.
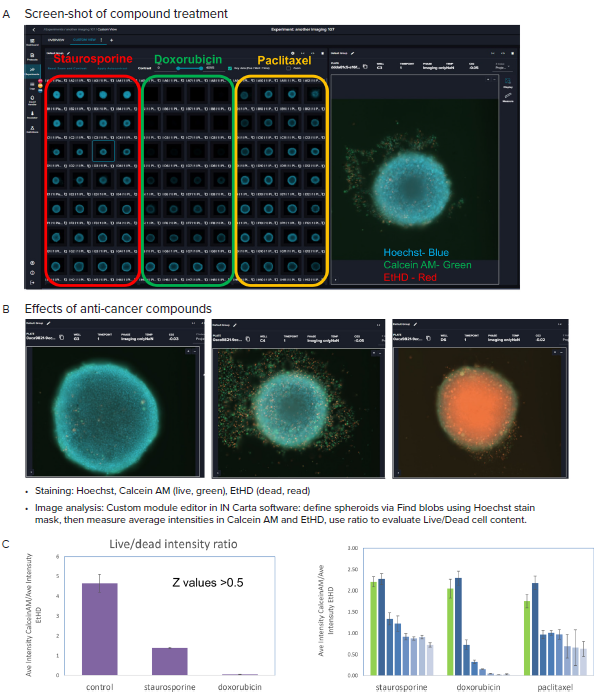
Figure 4. A, B Representative images (10X) of organoid culture taken after treatment with indicated compounds and staining. C. Graphical presentation of spheroid analysis: Ratios of the Ave Intensities of spheroids for Calcein AM and EtHD, maximum concentrations, and across concentration range. Averages and STDEV calculated from quadruplicates. Image Credit: Molecular Devices UK Ltd
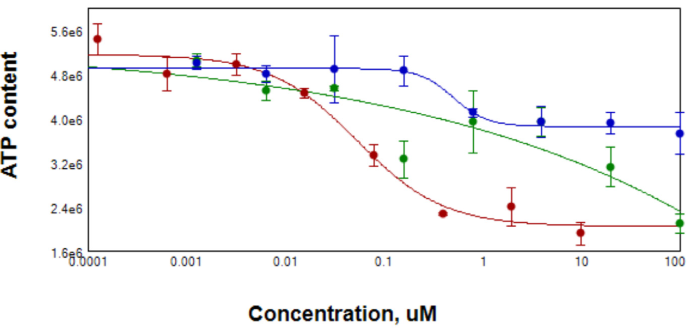
Figure 5. After compound treatment for 3 days, spheroid samples were tested for ATP content using CellTiterGlo reagent for 3D samples. EC50 for Staurosporin (red) was 0.05 μM, for Paclitaxel (blue) 0.5 μM. Data for Doxorubicin (green) were ambiguous due to the possible contribution of compound into Lumi signal. Image Credit: Molecular Devices UK Ltd
Summary
There is a significant unmet urgency for developing personalized and more effective approaches to treating cancer. The transition from 2D to 3D cell biology is widely seen as a game-changer in drug screening and cancer modeling because 3D models better represent the structure and functionality of tissue and have more predictive responses to drug effects. However, the complexity of 3D cell culture protocols interrupts cell lines' large-scale culturing and their various biomedical applications.
CellXpress.ai Automated Cell Culture System simplifies the process, including cell culture and assay set-up, cell culture imaging, screening, and data analysis. Automation enables 3D cell culture workflows to be reproducible at scale, largely increasing the confidence in drug screening readouts and creating insights for high-throughput drug discovery and precision medicine applications.
About Molecular Devices UK Ltd
Molecular Devices is one of the world’s leading providers of high-performance life science technology. We make advanced scientific discovery possible for academia, pharma, and biotech customers with platforms for high-throughput screening, genomic and cellular analysis, colony selection and microplate detection. From cancer to COVID-19, we've contributed to scientific breakthroughs described in over 230,000 peer-reviewed publications.
Over 160,000 of our innovative solutions are incorporated into laboratories worldwide, enabling scientists to improve productivity and effectiveness – ultimately accelerating research and the development of new therapeutics. Molecular Devices is headquartered in Silicon Valley, Calif., with best-in-class teams around the globe. Over 1,000 associates are guided by our diverse leadership team and female president that prioritize a culture of collaboration, engagement, diversity, and inclusion.
To learn more about how Molecular Devices helps fast-track scientific discovery, visit www.moleculardevices.com.
Sponsored Content Policy: AZO Life Sciences publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of AZO Life Sciences which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.