To adhere to Good “x” Practice (GxP) standards, laboratories subject to Good Manufacturing Practice (GMP) and Good Laboratory Practice (GLP) regulations must exhibit compliance with 21 CFR Part 11.
This entails effectively showcasing the adherence to quality assurance standards for microplate reader functionality and ensuring that data acquisition and analysis software aligns with Part 11 guidelines, ensuring overall data integrity in electronic records and signatures.
Molecular Devices is a frontrunner in offering comprehensive compliance solutions, including microplate detection systems and software. When paired with the validation services and support, the solutions provide a robust assurance of data integrity.
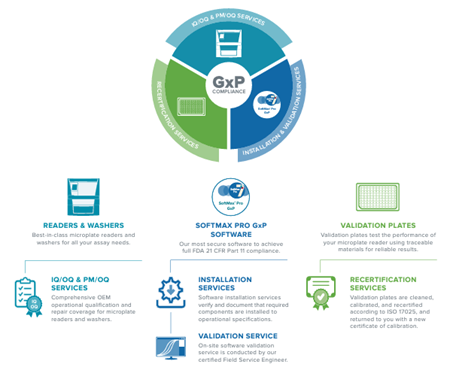
Image Credit: Molecular Devices UK Ltd
Introducing SoftMax® Pro 7.2 GxP Software – the cutting-edge, highly secure solution designed to attain comprehensive FDA 21 CFR Part 11 and EudraLex Annex 11 compliance. The streamlined workflows are meticulously crafted to guarantee data integrity, optimizing every step for simplified analysis and reporting with the microplate readers.
The team of experts is ready to collaborate with users, offering seamless setup for both single- and enterprise-level software configurations.
The company provides IQ OQ services through the validation package, ensuring users’ microplate readers achieve full compliance. Notably, the software incorporates significant data privacy and security enhancements, aligning seamlessly with the latest GDPR regulations.
What are the Benefits?
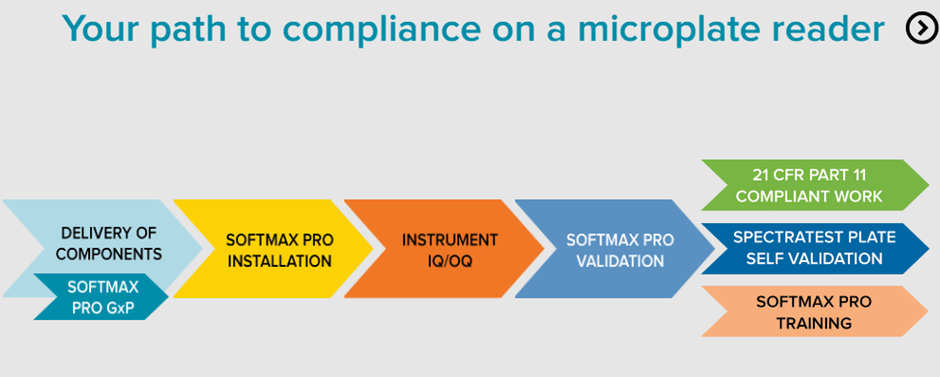
Image Credit: Molecular Devices UK Ltd
Track and Record all Changes
The system audit trail monitors all modifications, capturing details such as date and time stamps, usernames, user IDs, section statements, signature information, and read results.
Maintain Data Integrity
The digital document status system preserves data integrity by overseeing eSignatures and document workflows. Project teams can monitor the progression of documents as they navigate through stages of development, review, release, and controlled usage within a paperless environment.
Kickstart Users’ Compliance Journey with the “GXP Starter Bundle”
For researchers operating in GLP or GMP laboratories, users can attain complete GxP compliance effortlessly through an all-in-one solution:
SpectraMax Microplate Reader: Select from the comprehensive SpectraMax® series of user-friendly microplate readers.
SoftMax® Pro 7.1.2 GxP Software is the latest, highly secure software designed to meet full FDA 21 CFR Part 11 and EudraLex Annex 11 compliance, featuring streamlined workflows for maintaining data integrity.
The SoftMax® Pro Software Validation Package offers the most extensive documentation and tools for validating GxP administrator features, software operation, and analysis functions pertaining to microplate reader instrumentation.
Software Installation and Training: The software installation services ensure and document the installation of necessary components to meet operational specifications.