Scientists have known that interleukin-2 (IL-2), a signaling molecule, strongly influences the immune system.
However, attempts to leverage its potential for therapeutic purposes have gone futile due to serious side effects.
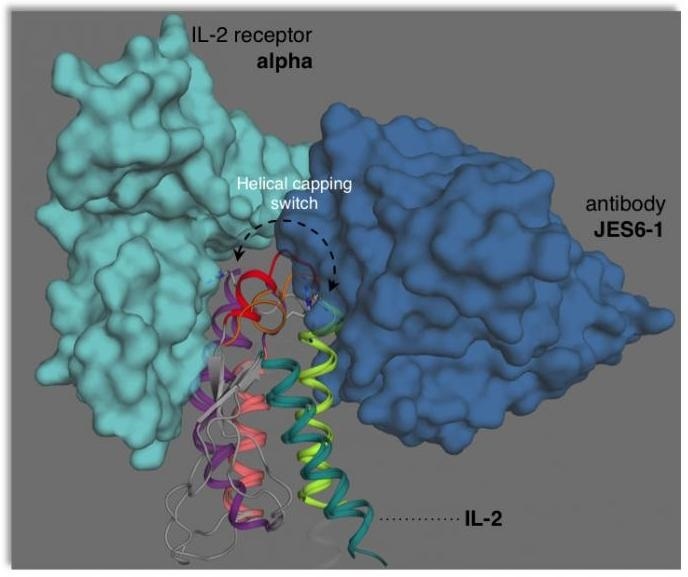
This illustration depicts how interleukin-2 (IL-2) adopts two different conformations that are “primed” for interactions with the JES6-1 antibody or the IL-2 receptor on T cells. This dynamical switching can be tuned to either boost or block immune responses depending on the desired therapeutic application. Image Credit: Viviane De Paula.
In a new study, researchers have investigated the complex interactions of IL-2 with receptor molecules on immune cells.
The study thus offers a layout for developing more targeted therapies to treat cancer or autoimmune diseases.
IL-2 serves as a growth factor to trigger the proliferation of T cell populations at the time of an immune response.
Various types of T cells have different roles to play, and IL-2 can trigger both effector T cells, directly attacking the immune system on particular antigens, and regulatory T cells, helping the immune system to take control once the risk is averted.
IL-2 can act as either a throttle or a brake on the immune response in different contexts. Our investigation used detailed biophysical methods to show how it does this.”
Nikolaos Sgourakis, Assistant Professor of Chemistry and Biochemistry, UC Santa Cruz
Sgourakis is a corresponding author of the study, recently published in Proceedings of the National Academy of Sciences. Viviane De Paula is the first author of the study and a visiting researcher in Sgourakis’ laboratory from the Federal University of Rio de Janeiro.
De Paula made use of nuclear magnetic resonance (NMR) spectroscopy to follow the structural dynamics of IL-2. The research was performed in close collaboration with researchers of the corresponding author Christopher Garcia’s team at Stanford University.
The researchers could demonstrate that IL-2 adopts two distinct structural forms (called conformations) that influence how it interacts with the receptors on T cells of various types.
In a solution, IL-2 naturally moves between a minor conformation and a major conformation. The researchers also demonstrated how specific mutations or interactions with other molecules can make IL-2 adopt one conformation or the other.
We have now come one step closer to a detailed understanding of how the IL-2 cytokine works. It is the first time that anyone has managed to observe a transient state of IL-2 directly. With the use of NMR, we were able to describe the structure, dynamics, and function of IL-2 in its two conformations.”
Viviane De Paula, Study First Author, Federal University of Rio de Janeiro
The study provides many possibilities to design drugs for stabilizing IL-2 in a specific conformation for therapeutic applications.
We can use this information to tweak the balance, depending on what we want to achieve in a clinical setting, To target regulatory T cells, we would want to stabilize the minor conformation, and to target effector T cells, we would want to stabilize the major conformation.”
Nikolaos Sgourakis, Assistant Professor of Chemistry and Biochemistry, UC Santa Cruz
Earlier attempts by other scientists had already proved that various monoclonal antibodies that target IL-2 could help proliferate different T cell populations in mice.
When combined with IL-2, one of the antibodies was found to be effective in treating inflammation and autoimmune disease in mouse models. At present, an identical human monoclonal antibody has been presented for clinical trials to treat autoimmune diseases.
The new study offers a realistic interpretation of these effects that can direct more drug discovery efforts.
According to Garcia, “The details of the mechanism we present offer a direct blueprint for drug discovery. Every drug company wants to know how to engineer this cytokine, and this paper provides some of the first bona fide structural clarity on the fascinating topic of IL-2 dynamics.”
Apart from De Paula, Garcia, and Sgourakis, the co-authors of the study are Kevin Jude and Caleb Glassman from Stanford and Santrupti Nerli from UC Santa Cruz. The study was financially supported by the National Institutes of Health.
Source:
Journal reference:
De Paula, V. S., et al. (2020) Interleukin-2 druggability is modulated by global conformational transitions controlled by a helical capping switch. PNAS. doi.org/10.1073/pnas.2000419117.