In a breakthrough study, a research team, headed by Northwestern University, has looked inside a human cell to visualize a multi-subunit machine that is responsible for controlling the expression of genes.
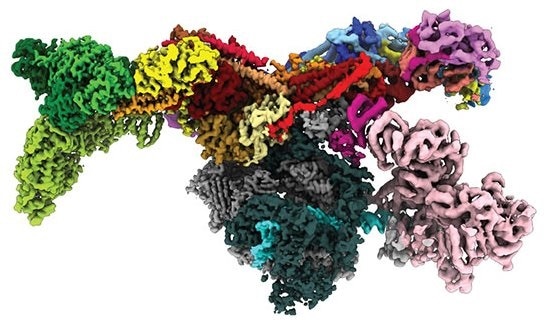
Image of the human Mediator-bound pre-initiation complex. Image Credit: Yuan He.
Dubbed the Mediator-bound pre-initiation complex (Med-PIC), the structure is an important factor in establishing which types of genes are triggered and which are inhibited. A Mediator helps place the rest of the complex—the general transcription factors and RNA polymerase II—at the start of the genes that the cell prefers to transcribe.
Using cryogenic electron microscopy (cryo-EM), the investigators viewed this complex in high resolution and this helped them to get a deeper understanding of how it works. Since this complex has a key role to play in various diseases, such as neurodegenerative diseases, cancer, metabolic disorders, and HIV, a new perspective of its structure can perhaps be exploited to treat diseases.
This machine is so basic to every branch of modern molecular biology in the context of gene expression. Visualizing the structure in 3D will help us answer basic biological questions, such as how DNA is copied to RNA.”
Yuan He, Study Senior Author, Northwestern University
“Seeing this structure allows us to understand how it works. It’s like taking apart a common household appliance to see how everything fits together. Now we can understand how the proteins in the complex come together to perform their function,” stated Ryan Abdella, the co-first author of the study.
The study was recently published in the Science journal on March 11th, 2021. This is the first-ever study in which the human mediator complex has been observed in 3D in the human cell.
Yuan He is an Assistant Professor of Molecular Biosciences in the Weinberg College of Arts and Sciences at Northwestern University. Abdella and Anna Talyzina, both graduate students at Yuan He’s laboratory, are the co-first authors of the study.
Renowned biochemist Roger Kornberg had discovered the specific Mediator complex in yeast back in 1990, a study for which he received the Nobel Prize in Chemistry in 2006. However, the Mediator contains as many as 26 subunits—56 in total when coupled with the pre-initiation complex—but scientists were able to obtain high-resolution images of the human version only now.
It’s a technically quite challenging project. These complexes are scarce. It takes hundreds of liters of human cells, which are very hard to grow, to obtain small amounts of the protein complexes.”
Yuan He, Study Senior Author, Northwestern University
There was a turning point when Yuan He’s team placed the specimen on a single layer of graphene oxide. By giving this support, the graphene sheet reduced the amount of specimen required for imaging. And in comparison with the regular support used—amorphous carbon—a kind of graphene helped enhance the signal-to-noise ratio for higher resolution imaging.
Once the sample was prepared, the researchers used cryo-EM, a comparatively new method that received the 2017 Nobel Prize in Chemistry, to find out the 3D form of proteins that are usually thousands of times smaller than the thickness of a human hair. The method works by sending a stream of electrons in a flash-frozen specimen to take several 2D images.
For this research work, Yuan He’s team recorded hundreds of thousands of images of the Med-PIC complex and they subsequently employed computational techniques to rebuild a 3D image.
Solving this complex was like assembling a puzzle. Some of those subunits were already known from other experiments, but we had no idea how the pieces assembled together or interacted with each other. With our final structure, we were finally able to see this whole complex and understand its organization.”
Anna Talyzina, Study Co-First Author and Graduate Student, Northwestern University
The resultant image revealed the Med-PIC complex as an elongated, flat structure that measures 45 nm in length. The team was also amazed to find that the Mediator travels with respect to the remaining of the complex, attaching to RNA polymerase II complex at a hinge point.
“Mediator moves like a pendulum. Next, we want to understand what this flexibility means. We think it might have an impact on the activity of a key enzyme within the complex,” concluded Abdella.
Source:
Journal reference:
Abdella, R., et al. (2021) Structure of the human Mediator-bound transcription preinitiation complex. Science. doi.org/10.1126/science.abg3074.