The BRCA1 protein known widely for its role in hereditary breast cancer is also a vital part of the cellular system repairing double-stranded DNA breaks. Recently, scientists from Japan identified a new fashion in which the cells protect the broken DNA ends to ensure that they are repaired correctly.
Protein Phosphatase 1 acts as a RIF1 effector to suppress DSB resection prior to Shieldin action. Image Credit: Osaka University.
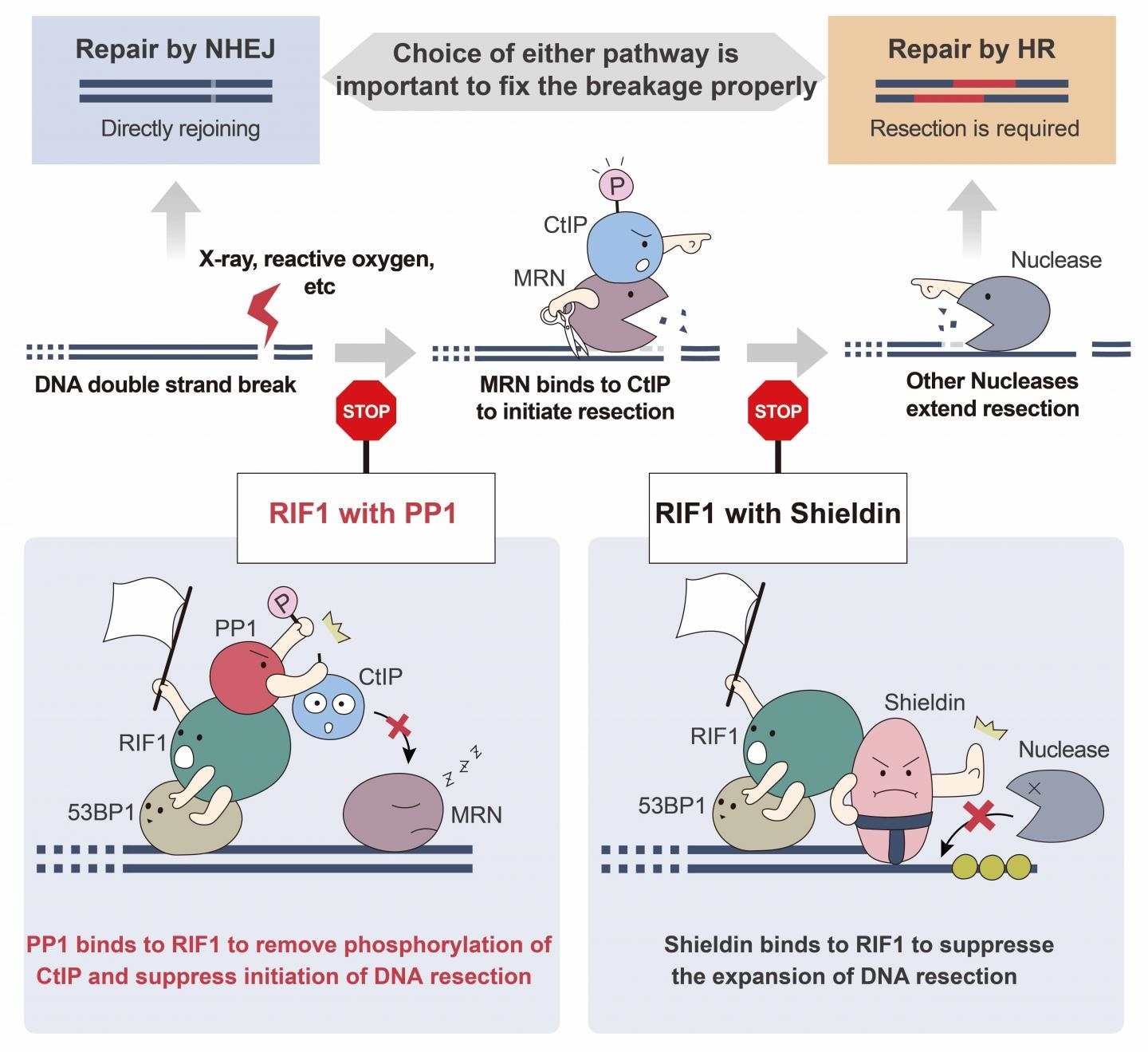
Osaka University researchers found that protein phosphatase 1 (PP1) binds to double-strand breaks at an early stage and initiates repair through a process called nonhomologous end joining rather than by homologous recombination.
The selection of a particular pathway from both processes to repair double-strand breaks is cautiously regulated by the cell in many different ways. In one process, the RIF1 protein binds to broken DNA ends, thereby preventing additional degradation by other proteins at the break site to repair by homologous recombination.
Double-strand breaks that are not protected by the RIF1 protein complex are susceptible to digestion by other proteins, which creates a section of single-stranded DNA for repair by homologous recombination. A protein called Shieldin can bind to this single-stranded DNA tail through RIF1 to prevent further digestion, but we suspected that other factors may also play a role in this process.”
Shin-Ya Isobe, Study Lead Author, Department of Biological Sciences, Graduate School of Science, Osaka University
The scientists employed a technique named proteomic mass spectrometry to determine various factors that protect the newly broken DNA ends and to discover other proteins interacting with RIF1.
We found that PP1 binds specifically to RIF1 at the broken DNA ends and that the physical interaction between these two proteins is necessary to block proteins that create single-stranded DNA from binding at double-strand break sites.”
Chikashi Obuse, Study Senior Author, Department of Biological Sciences, Graduate School of Science, Osaka University
The interaction between PP1 and RIF1 prevents the double-strand DNA breaks from developing a single-stranded “tail,” the binding place of Shieldin. This indicates that PP1 acts earlier in the process than Shieldin to push the cell toward the non-homologous end-joining repair pathway.
Our findings reveal a novel mechanism for selecting a double-strand DNA break repair pathway that acts early on in the repair process.”
Shin-Ya Isobe, Study Lead Author, Department of Biological Sciences, Graduate School of Science, Osaka University
The concerns with double-strand break repair are a vital feature of many cancers. Further knowledge on how the cell decides the pathway to fix damaged sites may offer a better understanding of cancer development. The outcomes of this research could help create new options to treat hereditary ovarian and breast cancer eventually.
Source:
Journal reference:
Isobe, S.-Y., et al. (2021) Protein phosphatase 1 acts as a RIF1 effector to suppress DSB resection prior to Shieldin action. Cell Reports. doi.org/10.1016/j.celrep.2021.109383.