Human cells are required to work similar to well-oiled machines to maintain the proper function of individuals’ body. Waste products, like carbohydrates, damaged cellular components, and misfolded proteins, get in the way and hence should be rapidly disposed of.
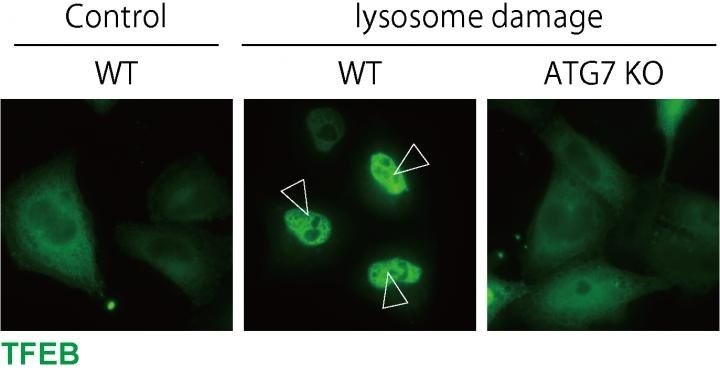
TFEB is localized to the nucleus upon lysosomal damage in WT cells, while ATG7 KO cells, in which LC3 lipidation is defective, show impaired TFEB nuclear localization. Image Credit: Osaka University.
Tackling this cellular “trash” are spherical, membrane-bound organelles known as lysosomes packed with a combination of potent enzymes. In a process known as autophagy, waste products are held inside a double-membrane vesicle—referred to as an autophagosome—that combines with a lysosome. The lysosomal enzymes subsequently function by disintegrating the waste into recyclable components.
However, if lysosomes are ruptured, their contents can escape and lead to serious damage to the cell. Kidney injury caused by calcium oxalate crystal and associated with the advancement of chronic kidney disease is actually the consequence of lysosomal damage caused by the crystals.
Hence, it is no wonder that cells have a number of pathways to repair or rapidly eliminate the damaged lysosomes. Yet the actual steps in these pathways and the way they interact during the lysosomal damage response are still vague.
In a new research published in the Nature Cell Biology journal, a research team headed by Osaka University has finally solved the interactions among the lysosomal damage response routes and established how they inhibit the oxalate caused by kidney injury.
A protein called TFEB turns on genes necessary for autophagy and the production of new lysosomes in response to lysosomal damage. By inhibiting TFEB function in HeLa cells and then inducing lysosome damage, we confirmed that TFEB is activated upon lysosomal damage and is necessary for the removal of damaged lysosomes.”
Shuhei Nakamura, Study Lead Author, Osaka University
Binding of lipids to a protein known as LC3 is a critical step in autophagosome formation. To their amazement, the scientists also observed that lipidated LC3 was essential to trigger the TFEB during the lysosomal damage response; however, there was no clear connection between the systems.
Calcium is a known activator of TFEB. To identify how the TFEB and LC3 systems overlapped, we investigated lysosomal calcium channel TRPML1. We found that lipidated LC3 was recruited by lysosomes in response to damage, and that the lipidated protein interacted with TRPML1, causing increased calcium efflux from the lysosome, which activated TFEB.”
Tamotsu Yoshimori, Study Senior Author, Osaka University
The physiological significance of this interaction was subsequently established using a mouse model of oxalate crystal-induced kidney damage. TFEB-lacking mice had more severe kidney damage when compared to the control animals. Interpreting the interactions of the pathways is the first step to prevent lysosomal damage-associated diseases.
Source:
Journal reference:
Nakamura, S., et al. (2020) LC3 lipidation is essential for TFEB activation during the lysosomal damage response to kidney injury. Nature Cell Biology. doi.org/10.1038/s41556-020-00583-9.