Overview
MatTek’s patented EpiDerm system is a first-in-class in vitro testing technology for formulation scientists and dermal toxicologists.
With the availability of several ECVAM validations and OECD-accepted test guidelines, EpiDerm is a tested in vitro model system for pharmaceutical, chemical, and skin care product testing.
MatTek’s patented EpiDerm system is known to be the foremost in vitro testing technology that has been utilized by formulation scientists and dermal toxicologists. With the availability of multiple ECVAM validations and OECD-accepted test guidelines, EpiDerm is a proven in vitro model system for pharmaceutical, chemical, and skin care product testing.
Key features
- Skin Irritation (OECD TG 439), Skin Corrosion (OECD TG 431), and Phototoxicity (OECD TG 498)
- Outstanding in vitro–in vivo correlation
- Produced under GMP
Technology
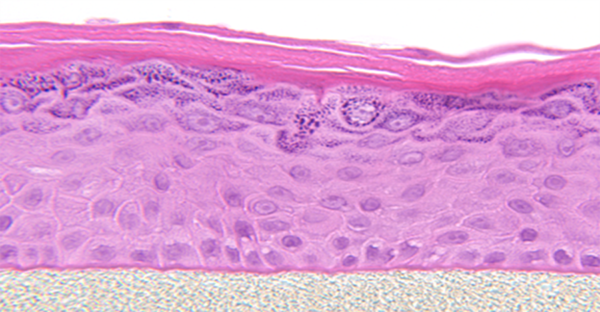
Image Credit: MatTek
The EpiDerm system also known generically as a Reconstructed Human Epidermis (RHE), is a user-friendly, highly differentiated 3D tissue model comprising normal, human-derived epidermal keratinocytes (NHEK) cultured on uniquely made tissue culture inserts.
Having cultured at the air-liquid interface (ALI), EpiDerm enables the assessment of topically applied chemicals, compounds, cosmetic or personal care product ingredients, and ultimate formulations.
With the availability of straight protocols and 20 years of data, EpiDerm is known to be the gold standard for a variety of highly predictive in vitro applications.

Tissue Culture Insert. Image Credit: MatTek
Image Credit: MatTek
EpiDerm displays human epidermal tissue structure and cellular morphology with higher reproducibility and uniformity. It is a 3D structure comprising spinous and granular layers, organized and proliferative basal cells, and cornified epidermal layers that are active mitotically and metabolically.
Human relevance
With the availability of multiple ECVAM validations and OECD OECD-accepted guidelines, EpiDerm is considered to be a proven in vitro model system that has been utilized for chemical, pharmaceutical, and skin care product testing.

Image Credit: MatTek
Applications
The EpiDerm 3D human tissue model has been utilized throughout an extensive range of applications such as safety and risk assessment, as well as biological efficacy.
With the availability of air-liquid Interface (ALI) culture technology, EpiDerm could be utilized to assess biological responses to topical applications of ingredients or formulations. Easy protocols and the evaluation of early cellular endpoints enable research to obtain data in just days, not weeks or months.
Skin irritation (OECD TG 439)
Make use of EpiDerm to identify the skin irritation potential of solids, liquids, gels, lotions, ointments, or creams. Tissue viability outcomes have been obtained within three days.
Skin corrosion (OECD TG 431)
Users can identify the skin corrosion potential of test materials in one single day.
Skin hydration
Users can quantify the electrical impedance of EpiDerm to evaluate the efficacy of topically applied moisturizers such as gels, creams, and lotions. The Skin Hydration protocol enables data analysis within 24 hours.
Dermal drug delivery
Utilize existing transdermal permeation equipment or MatTek’s simple single-insert permeation devices to assess API permeation and flux. Determine relative safety with the EpiDerm MTT ET-50 assay.
Phototoxicity (OECD TG 498)
Determine the phototoxic effects of topically employed test substances with the help of the EpiDerm Phototoxicity Assay.
Using the EpiDerm tissue model, users can find the effects of systemically applied test substances.
Dermal genotoxicity
It is possible to determine the genotoxic effects of topical or systemic test substances using EpiDerm Genotoxicity Assays (pre-validated)
Epidermal differentiation
Calculate active compounds for effects on epidermal differentiation with the help of the under-developed EpiDerm tissue model